| UHH > Faculty of Biology > Teaching Stuff > Highlights of Biochemistry > Anthrax | search |
Bacillus anthracis produces a toxin composed of three distinct poteins. These are termed (for historic reasons) protective antigen, oedema factor, and lethal factor. Protective antigen is secreted in a precursor form which can heptamerize and form a channel in membranes, which allows the other two factors to enter the target cell. Oedema factor is an adenylate cyclase which leads to an impairment of host defenses. Lethal factor is a protease causing the lysis of macrophages. The concerted action of these proteins is in line with other microbial toxins.
Invasion of the target cell by the anthrax toxin is a well organized procedure. First, protective antigen (PA) binds to a receptor on the cell's surface. Next, from the 83 kD PA molecule a Domain 20 kD in size is cleaved off by a host protease, furin. Third, seven of the shortened (and still receptor bound) PA63 assemble to a ring-like structure. This complex is able to bind either oedema factor (OF) or lethal factor (LF). The next step is an endocytosis: the cellular membrane containing the receptor bound toxin complex is tilted inward to eventually form a vesicle with the toxin bound to the inner surface. The lumen of this vesicle is acidified (like a lysosome), which induces a conformational change of PA63. This leads to an insertion into the membrane with pore formation. Through this pore OF or LF pass from the endosome into the cytoplasm of the cell, ready to start their destructive enzymatic work.
Anthrax toxin receptor (ATR)
| Integrin aplpha2 ( I domain ) |
The human cellular receptor for PA has been identified. It occurs more than tenthousendfold on the surface of macrophage cell lines cells. A strong sequence similarity exists to the I domain of subunit alpha of integrin alpha2beta1, to the von Willebrand factor A domain consensus sequence, and a protein upregulated in human colorectal cancer endothelium termed TEM8. ATR and TEM8 are supposed to originate from the same gene by alternative splicing. A truncated, soluble form of ATR (lacking the membrane anchoring sequence) is able to protect cell cultures against the lethal action of anthrax toxin. ATR is expressed in a variety of tissues including the central nervous system, heart, lung, and lymphocytes. The ATR cDNA codes for a Protein of 368 amino acids. It is predicted to have a 27 amino acid leader sequence, an extracellular domain of 293 amino acids, a 23 residues transmembrane region, and a short cytoplasmic tail at the carboxy terminus.
Integrin alpa2beta1 is the collagen receptor on platelets and fibroblasts as well as the receptor for echovirus-1, a human pathogen. ATR is supposed to exhibit the same folding pattern as the integrin alpa2 I domain, which is shown here.
Protective antigen
| Anthrax protective antigen ( soluble form ) |
| structural details |
PA is produced by the bacteria as a protein of 764 amino acids (Mr 85810). Cleavage of a 29 residue signal sequence releases the mature PA83. In this state PA is inactive except for its binding affinity for ATR. Only after the furin-mediated cleavage to form PA63 binding of one of the other toxin components is possible. Heptamerization and pore formation after acidification leads to a structure similar to the staphylococcal alpha-hemolysin. The pore formed is cation-selective and able to pass other molecules as well, as e.g. the diphtheria toxin A chain. The structural details shown here are of the secreted mature PA83 (renumbered here 1-735).
Lethal Factor
| Anthrax lethal factor |
| structural details |
LF is a zinc dependent metalloprotease of 809 amino acids (minus 33 signal sequence). In the cytosol of affected cells the protease cleaves dual specificity mitogen activated protein kinase kinases (MAPKK). The cleavage of this class of proteins occurs in the N-terminal proline-rich region preceding the kinase domain. Protein-protein interactions neccessary for the assembly of signalling complexes are thus disrupted.
Oedema Factor
OF is an adenylyl cyclase that is activated by calmodulin. Upon activation a dramatic elevation of host cell cAMP levels are found. The protein is 800 aminoacids (minus 33 signal sequence) in size. No detailed structural information is known to this date.
Tackling anthrax
As other bacilli Bacillus anthracis is able to differentiate into dormant spores, which may last for years in spite of adverse environmental conditions. The spores will germinate to vegetative cells as soon as nutrients are available. This may occur on the skin or within the lung of a human or animal. Once inside a body the bacilli grow to high titers, aided by the toxin in overcoming host defense. Besides the toxin other components (a poly-D-glutamic acid capsule) contribute to virulence. Both capsule and toxins are coded on plasmids harboured by the bacteria.
Protection against infection may be gained by vaccination. Licenced vaccines are spores from toxigenic but nonencapsulated B. anthracis or aluminum hydroxide adsorbed cell-free PA. The use of the attenuated live vaccines may have local adverse responses and are not very effective. A still experimental vaccine was constructed by engineering the PA gene into an originally plasmidless bacterial strain. Human vaccination is not usually done as natural anthrax infections are rather rare.
In early stages infections are cured by antibiotics, with ciprofloxacin as drug of choice. Unrecognized infections usually are fatal. Anti-toxin treatment (e.g. with immunglobulins directed against PA or synthetic peptides competing for binding of the toxin factors) may help to overcome a severe infection.
| Ciprofloxacin | |
| formula | model |
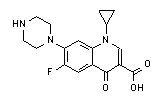 |
|
Literature:
KA Bradley et al, Identification of the cellular receptor for anthrax toxin, Nature 414 (2001) 225-229
AM Friedlander, Tackling anthrax, Nature 414 (2001) 160-161
S Cohen et al, Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax, Infect. Immun. 68 (2000) 4549-4558
M Mourez et al, Designing a polyvalent inhibitor of anthrax toxin, Nature Biotechnol. 19 (2001) 958-961
J Emsley et al, Crystal structure of the I domain from integrin alpha2beta1, J. Biol. Chem. 272 (1997) 28512-28517