Covalent bonds are the most important type of bond occurring in organic molecules. They are characterized by a common pair of electrons between two neighbouring atoms. Depending on the involved partners, simple, double or triple bonds can be formed (H-H, O=O, N=N).
The delta G of a covalent bond is around -50 to -100 kcal/mol (-210 to -420 kJ/mol), i.e. a considerable amount of energy is stored in it. This does not necessarily mean that the respective molecules are in a state of high energy. They may even be said to be in a state of low energy since the energy is used to maintain the bonds. In cells, enzymes participate in the formation and breaking of covalent bonds.
Beside the just mentioned strong covalent bonds, molecules form also weak interactions (also called supplementary valences) of different kinds. The free energy (G) of these interactions ranges between -1 and -7 kcal/mol ( -4 to -30 kJ/mol) and is thus only slightly stronger than that of thermal molecular movements (-0.7 to -3 kJ/mol). Under physiological conditions, weak interactions are continuously formed and broken again having an average half-life of just some fractions of a second. Enzymes are usually not involved in these processes.
Molecules have the tendency to develop the strongest interactions possible under the given conditions. Weak interactions are so important, because they add up and thus generate very strong molecule conformations. The secondary, tertiary and quaternary structure of proteins, the double helix of the DNA, the membrane structures and complex intracellular particles like the ribosomes are all maintained by weak interactions. The important thing here is the shape of the reactants. The more complementary the structures, the better their fit, the more weak interactions can form and the more stable is the resulting conformation or aggregate.
Ionic Interactions. This type of interaction is maybe the one most rightfully termed bond. Its delta G is around -5 to -7 kcal/mol (-20 to -30 kJ/mol). The acting forces are of electrostatic nature and develop between ions with opposite charges (cations and anions, ionized bases and dissociated acids). They may develop considerable strength in non-water surroundings. Due to their existence, a Na+Cl- crystal, for example, has the very high melting point of 801°C.
Hydrogen Bonds. Water is an electrostatic solvent. The electrons of a water molecule are distributed unevenly, since they are more strongly attracted by the oxygen atom than by the two hydrogens. Electronegative atoms, like oxygen, have a tendency towards polarity and supply the molecule with a directed dipole moment.

WATER. To the left: "ball and stick" model. The positioning of the atoms becomes clear (red: oxygen, green: hydrogen. In most other images of Botany online , hydrogen is given in turquoise). In the middle: Distribution of electron density. The electron clouds correspond to the van-der-Waals radius of the single atoms. The radius of hydrogen is 1.2 Å, that of oxygen 1.4 Å. The length of the covalent bonds between O and H is 0.95 Å. The uneven charge distribution causes a dipole momentum
To the right: Solid model.
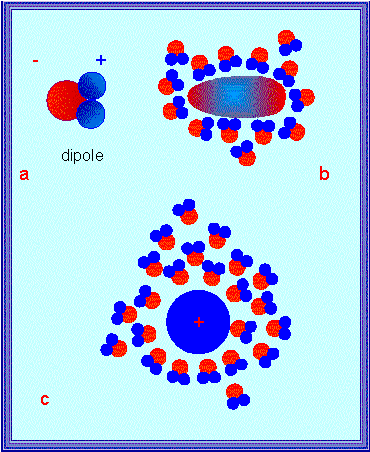
Dipole properties of a water molecule: Formation of hydration sheath and orientation of the water molecules within a hydration sheath in dependency on the charge distribution within the hydrated molecule. - Hydrogen bonds between water molecules (below)
|
|
The directed dipole moment causes a partially ionic character (due to the uneven charge distribution within the molecule) creating an affinity between the negative part of a molecule and the positive part of its neighbour. Such an interaction is called a hydrogen bond. It is by no means restricted to water alone, but develops wherever a small electronegative atom (like oxygen, nitrogen or fluorine) and a hydrogen atom that is bound covalently to another electronegative atom are close to each other. Examples are:
-O-H . . . . O==N-H . . . . O=C-
-N+ -H . . . . O=
Macromolecules may develop both inter- and intramolecular hydrogen bonds. They are strongest, if the involved atoms are arranged linearly. The strength of their interactions drops drastically as soon as angles appear between the affected groups. The length of hydrogen bonds depends on the type of the involved groups and is between 2.7 and 3.1. Å.
Hydrogen bonds are the reason for the high melting and boiling points of water. The shape of the molecule and the structure of the weak interactions causes the crystal structure of ice and even in liquid state exists still a high degree of order with the water molecules arranging in the form of micelles.
The polar character of water is mirrored by its high dielectric constant (80 at room temperature). It says that two electrical charges of opposite signs attract each other in water with just 1/80th the force that they would exert on each other in air or in a vacuum. This explains why ions detach themselves so much easier from their crystal structure in water than in air. The thermally caused molecular movements at room temperature are thus sufficient to overpower the relatively weak attractions of the ions within the crystal and to let them dissociate into the aqueous medium. Water molecules display a strong tendency to form shells of hydration. In this way, they shield ionic charges and neutralize them partially. Water molecules orient themselves within the electrostatic field and are thus immobilized.
Water dissociates to a slight extent producing hydrogen ions (H+ or rather H30+) and hydroxyl ions (OH- or H3O2-), that again may form ionic bonds with other ionic solutes.
Van der Waals attraction is the term used for non-specific attractions between two atoms that are close to each other. These interactions depend on the distance between the respective atoms (or atom groups or molecules). At too close distances, repulsive forces are dominating (overlapping of electron shells). The energy of Van der Waals attractions is only slightly higher than that of thermal molecular movements: -0.7 to -1 kcal/mol ( -3 to -4 kJ/mol). Consequently, Van der Waals attractions are under physiological conditions only of importance, if as many atoms of a molecule as possible are engaged. They are strongest, if the involved molecular structures complement each other. Van der Waals attractions are additive and have thus a much greater impact on macro- than on small molecules.
The coupling of a substrate to an enzyme is to a large extent stabilized by Van der Waals attractions. The stronger they are, the higher is the affinity between an enzyme and its substrate. An energy of 2-3 kcal/mol (8-12 kJ/mol) is normally enough to guarantee sufficient selectivity. But the energy attracting enzyme and substrate should not be too great, since a quick turn-over ratio is only achieved by fast binding and releasing of the substrate (or the formed product).
|
|