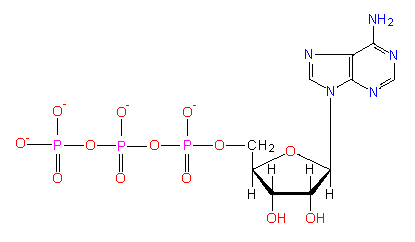
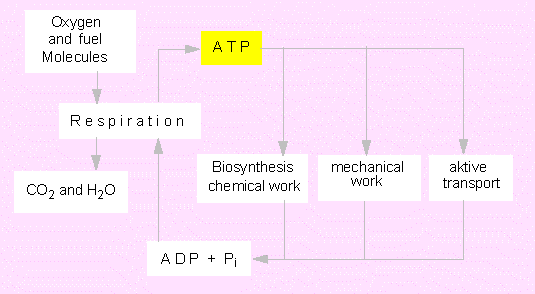
ATP is regarded as a universal source of energy occuring in all cell types. It is produced mainly during the oxidation of energy-rich (reduced) compounds processed in the respiratory chain and in photosynthesis. ATP is needed
ATP occurs usually as a magnesium or a manganese salt. For
its hydrolysis, magnesium ions are necessary. Whenever it is
spoken of ATP-degradation, it is always the hydrolysis of the
terminal phosphate group(s) that is meant. The reactions are
reversible:
ATP + H2O < > ADP + H3PO4 (= Pi)
or
ATP + H2O < > AMP + pyrophosphate (= PP)ADP + H2O < > AMP + Pi
ADP and AMP are the abbreviations for adenosine diphosphate and adenosine monophosphate.
The phosphates are linked anhydrously, the innermost phosphate residue and the sugar residue are linked by an ester bond. Hydrolysis depends on the pH. The delta G° is -7.3 kcal/mol (ca -30.6 kJ/mol) at pH 7, i.e. at almost physiological conditions. It increases with a rising pH and is -10 kcal/mol (ca -42 kJ/mol) at pH 9.
Since the delta G° for the breakdown of a
pyrophosphate and for that of one phosphate residue are roughly
the same, ATP, ADP and AMP can rather easily be converted into
each other:
ATP + AMP < > 2 ADP
The delta G of the ATP breakdown is not very high compared to other phosphorylated compounds. Under this aspect, the term 'energy-rich linkage' seems irritating, but it has gained acceptance in biochemical literature as its hydrolysis is easily performed (with the help of the respective enzyme) and the energy is actually useable. The reason for the rather easily broken down linkage is in the electron accumulation at the terminal phosphate residues. Identical charges (here they are negative) repel each other and are in this case neutralized by hydrolysis.
In many cases, the terminal phosphate residue that is
cleaved off from the ATP is not given away into solution as a
free inorganic phosphate, but is transferred onto another
molecule that becomes consequently phosphorylated. This process
works also the other way round: a phosphorylated compound with
a delta G° > -8 kcal/mol (-34 kJ/mol) can
transfer its phosphate residue to ADP that as a consequence
becomes ATP.
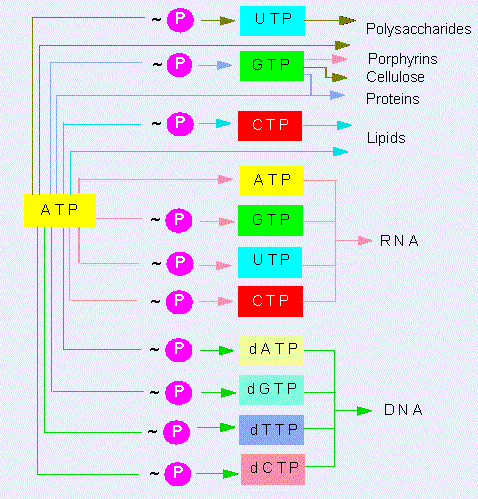
Besides the adenosine nucleotide phosphates, uracil, cytosine and guanine phosphates occur, too:
UMP, UDP, UTP, CMP, CDP, CTP, GMP, GDP, GTP.
The triphosphate nucleosides of these compounds and those of
ATP are components of RNA. They are integrated into the polymer
by splitting off pyrophosphate ( = PP). The corresponding
desoxyribose derivatives (dATP, dGTP, dCTP....) are necessary
for DNA synthesis, where dTTP is used instead of dUTP. The
terminal phosphate residues of all nucleoside di- and
triphosphates are equally rich in energy. The energy set free
by their hydrolysis is used for biosyntheses. They share the
work equally: UTP is needed for the synthesis of
polysaccharides, CTP for that of lipids and GTP for the
synthesis of proteins and other molecules. These specificities
are the results of the different selectivities of the enzymes,
that control each of these metabolic pathways.