P-proteins (phloem proteins) are typical components of the phloem. They occur in sieve tubes and aggregate in fibrils, tubules, membrane-like or paracrystalline structures, lamellas, flakes and other structures. They have been isolated from the exudate of sieve tubes. Pumpkin (Curcubita) has become a favourite specimen, because its sieve tube content is under considerable pressure and flows easily out after cutting (R. KOLLMANN, at that time at the Botanisches Institut der Universität Bonn, 1960; H.-D. BEHNKE and I. DÖRR, the same institute, 1967; J. CRONSHAW and K. ESAU, University of California, Santa Barbara, 1967). P-proteins do not bind myosin, they are not contractile and vinblastin and colchicine exert no influence on them. Molecular weights of 15,000, 28,000, 59,000, 116,000 and 220,000 Daltons have been determined, all display a strong aggregation behaviour. The proteins are very alkaline and differ depending on their origin. Even in closely related species like Cucumis melis and Cucumis sativus have differences been found.
Their function remains largely a mystery. It is questionable, too, whether they can be discussed side by side with cytosceleton systems, since a participation in transport processes or the determination of certain cellular structures could not be proven. Solely their polymerization and aggregation behaviour, respectively could justify their discussion in this context.
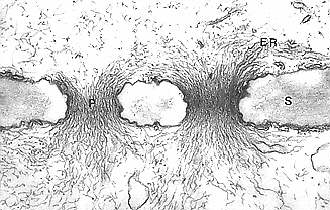
P-protein filaments (P) in the pores of a sieve plate (S); ER: endoplasmatic reticulum (Aristolochia brasiliensis) H.-D. BEHNKE, 1971
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de