Since all biological processes are assumed to be accomplished by the interaction of molecules, a theory of biological pattern formation has to describe the change of a substance concentration in space and time as function of the concentrations of the other substances involved. According to our theory, a simple molecular interaction with pattern-forming capability would consist of an "activator" that has a non-linear positive feedback on its own production rate. Its autocatalysis is slowed down by a long ranging "inhibitor" (for equations, see Gierer and Meinhardt, 1972, Meinhardt, 1982,1998). A necessary condition for the formation of a stable pattern is that the inhibitor diffuses much faster than the activator and has a shorter half life. In other ranges of parameters oscillations and travelling waves can occur. These modes will be discussed further below in connection with the patterns on the shells of molluscs.
The simulations shown in Fig. 2 and 3 demonstrate that the activator-inhibitor reaction has properties basic for the explanation of biological pattern formation. A pattern emerges whenever the size of the field becomes larger than the range of the activator. In fields with a size comparable to the activator range, the high activator concentration can be formed at one end of the field only. This is a very important aspect for the understanding of pattern formation in an early embryo: one side of the embryo becomes different from the other. By the graded activator and/or inhibitor distribution, different genetic information can become activated in different parts of the tissue in an ordered sequence (see below). Thus, the system is able to generate "positional information".
It is only recently that pattern forming systems have been uncovered that follow this type of regulation. In the Drosophila fly the precursor cells of the peripheral nervous system (neuroblasts) are derived from two lateral bands of ectodermal cells. Crucial for neuroblast formation are the genes of the Achaete-Scute complex. Both genes have a direct autocatalytic feedback on their own synthesis (Van Doren et al., 1992, Culi and Modolell, 1998). Cells that express these genes activate the gene Delta that codes for a molecule exposed at the cell surface. Its stimulates the receptor Notch, a molecule ubiquitously present on all cells in that region and that acts ultimately on the Achaete-Scute complex in an inhibitory way. In this way, particular cells are singled out to participate in the formation of the peripheral nervous system while the remaining cells form the ectoderm. Although Delta cannot diffuse, experimental evidence suggest that the range of this inhibition goes beyond adjacent cell, but the corresponding mechanism is not yet known.
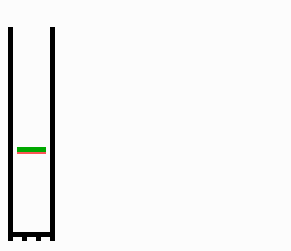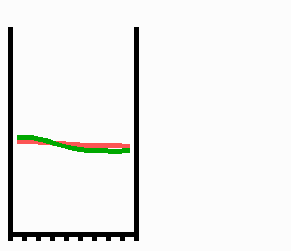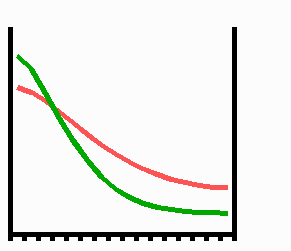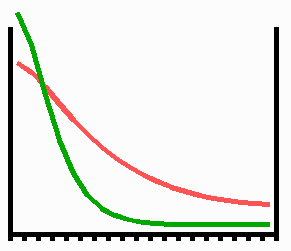
Fig. 2: Pattern formation by interaction of an autocatalytic activator (green) and its long ranging antagonist, the inhibitor (red). Assumed is a linear array of cells that grows at both margins. If a critical size (the range of the activator) is exceeded, random fluctuations are sufficient to initiate pattern formation. A high concentration appears at one marginal position of the field since a central maximum would require space for two activator slopes for which no space is available at the critical size. A graded concentration profile results that can be maintained upon further growth.
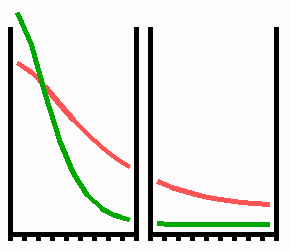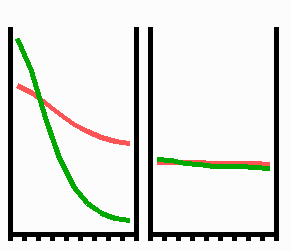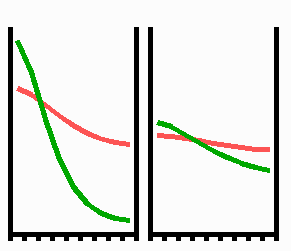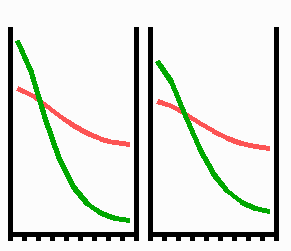
Fig. 3: Regeneration: With the removal of the activated region, the source region of the inhibitor is also removed. After the decay of the remnant inhibitor (red), a new activator maximum (green) regenerates.
An important feature of many developing systems is their ability to regenerate. For instance, in the freshwater polyp Hydra, after removal of a head a new head regenerates. This is a property of the activator-inhibitor system. With the removal of the activated region, also the inhibitor-producing cells are removed. The remnant inhibitor decays until a new maximum is formed via autocatalysis (Fig. 3). However, the actual pattern formation in Hydra is more complex since also the signal for tentacle and foot formation must be generated in a precise spatial relation to each other (see Fig. 7).