Since antiquity belongs the folding up of the pinnate leaflets and leaves of some plant species (especially from the leguminosa group and the genus Oxalis) after touching or vibration to the most fascinating phenomenons of botany. These plants are called sensitive. The folding up of the two leaf halves of the Venus’ flytrap (Dionaea muscipula), the tentacle movements of the Drosera-species belonging to the same family, the movements of the filaments of certain Centaurea and Berberis species, and the movements of the stigma halves of Mimulus are comparable reactions.
Explanations for these phenomenons were looked for already quite early. Just as in all cases discussed up till now have the movements and the stimulus perception, conduction, and conversion to be regarded as separate processes. It suggested itself to compare the movements of the leaves of Mimosa with the circadian movements of some leaves, especially since they can be very impressively observed in certain leguminosa.
R. H. J. DUTROCHET (1776-1847, doctor and scholar at Touraine and Paris) suspected that the rising and lowering of the leaves is based on an antagonism of upper and lower joint halves (1837). Direct measurements carried out by the physiologist E. W. von BRÜCKE in 1848 showed that the turgor in the pulvinus rises when the leaves take up their sleeping position, but this increase is restricted to the upper parts of the pulvinus alone. This proved that the observed movements were actually turgor movements. Supplementing examinations of J. v. SACHS (1859) and W. PFEFFER (1873) substantiated these results.
Plant anatomical studies showed that the flexibility is reduced to the joints where the vascular bundles that run in leaf stalks and shoots in the periphery are joint in a central strand surrounded by large cells of the cortex parenchyma. They are predestined to change their turgor by reversible water uptake. The high elasticity of their cell walls is a further precondition for deformation. The cells of the upper pulvinus are able to increase their turgor far more than cells usually can. The cells of the lower side loose water very easily and are thus especially deformable.
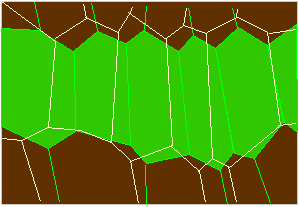
Scheme of the concertina-like tissue at the lower side of the primary joint of Phaseolus-cells in a fully turgescent (green) or a relaxed state (outlines; according to W. HAUPT, 1977).
A concerted movement presupposes a well-tuned interaction of the two antagonistic pulvinus halves. The measurements of BRÜCKE have already shown that this is actually true.
The situation is similar with the pulvinus between the two leaf halves of Dionaea muscipula. The upper side of each leaf half is equipped with three sensory bristles that perceive the stimulation. The examination of their anatomical structure suggests that the movement is caused by a leverage since a group of large deformable cells are located at the base of the bristles.
That we deal with nastic movements here, comes from the anatomy of the tissues of movement (dorsiventral structure). Due to the reaction towards vibration is it spoken of seismonasty. Reactions caused by touching are generally referred to as thigmonasties. Without doubt has seismonasty to be regarded as a special case of thigmonasty that is characterized by an especially fast conversion of the stimulus. When single pinnate leaflets of the Mimosa vibrate or are touched, do they fold up in pairs. The reaction starts at the point of touching and moves successively over the whole pinnate leaf, is passed on to the next pulvinus and from there further on. Two important conclusions can be drawn:
- The reaction runs either completely or not at all.
- It is reversible. The leaves return to their starting position after 15 to 30 minutes.
In any case exists some kind of stimulus forwarding in a leaf resulting in our question how it works. Reactions of this type are known from animal neurones. They conduct the stimulus along the axon of the neurone by electrochemical means. In other words: the membrane is locally depolarized and the depolarization spreads with high velocity to neighbouring regions. The structure of the axon guarantees a directed spreading. Do such membrane depolarizations also take place in the mimosa (and in the irritable organs of other species like Dionaea, Drosera, Berberis, etc.)? Hints exist that this is indeed the case, although it is extraordinarily difficult to measure changes of the membrane potential of plant cells experimentally. Measurements from cells of other species, especially from the giant cells of the alga Nitella, but also from cells of the tissue of movement of the mimosa show the following:
Since the concentration of certain ions differs from outside to the inside of a cell due to different selective permeabilities, develops a measurable membrane potential. Potassium ions, for example, diffuse along their concentration gradient from the inside to the outside of the cell thus causing a separation of charges since their counterions (usually chlorid-ions) can either pass the membrane in very small amounts only or not at all. Compared to the total concentration is the amount of ions moved via the membrane extraordinarily small. The resting potential of a plant is about –150 to –200 mV (Nitella, Elodea, Vallisneria). During an action potential rises the membrane’s permeability for chloride-ions that pour out according to their concentration gradient thereby depolarizing the membrane. This depolarization again increases the flow of potassium ions (phase of repolarization). This phase occurs not due to an active uptake of ions.
A stimulation of the cell (however it occurs) causes always a transient local breakdown of the potential due to a short-term permeability of the membrane for ions. The pouring in of cations brings about a transient rise of the potential (also called action potential) that returns to its old state immediately after having passed its maximum. The period until the value of the resting potential is re-established is called the refractory period. During this time can the respective part of the membrane not be stimulated. The depolarization spreads fast and very easily (during milliseconds) to the other membrane parts of the cell and do thus effect its whole membrane. The action potential of one cell may stimulate neighbouring cells if it is high enough. The only necessary precondition is the passing of the threshold of stimulation of these cells. This explains on one hand the conduction of stimulation from cell to cell, and on the other hand also why a cell reacts either completely or not at all.
Cells of mimosa’s tissue of movement cannot only be stimulated mechanically but also electrically, a proof for the existence of an electrical stimulus forwarding. In addition could it be observed that stimulated cells secrete a substance that is spread by the transpiration stream and that reaches in this way also the pulvinus and causes them to react.
Electrical and chemical stimulation and forwarding of stimulation differ in their velocity. Depending on the organ is it 0.76-26 cm/sec for electrical and 0.15-2 cm/sec for chemical forwarding of stimulation. Both types supplement each other. The chemical forwarding of stimulation is of advantage wherever electrical resistances occur, for example in a pulvinus or in dead cell areas.
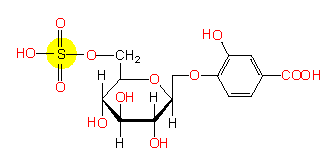
A stimulating substance from mimosa