The biochemistry supporting life on earth depends in terms of gain of energy on oxidative reactions. The final product of metabolic pathways based on carbon is carbon dioxide which is released into the atmosphere. To close the carbon cycle it is neccessary to feed the carbon dioxide back to the food chain. The only enzyme capable of this task is ribulose-1,5-bisphosphate carboxylase (rubisco). It comprises the starting point of any food chain.
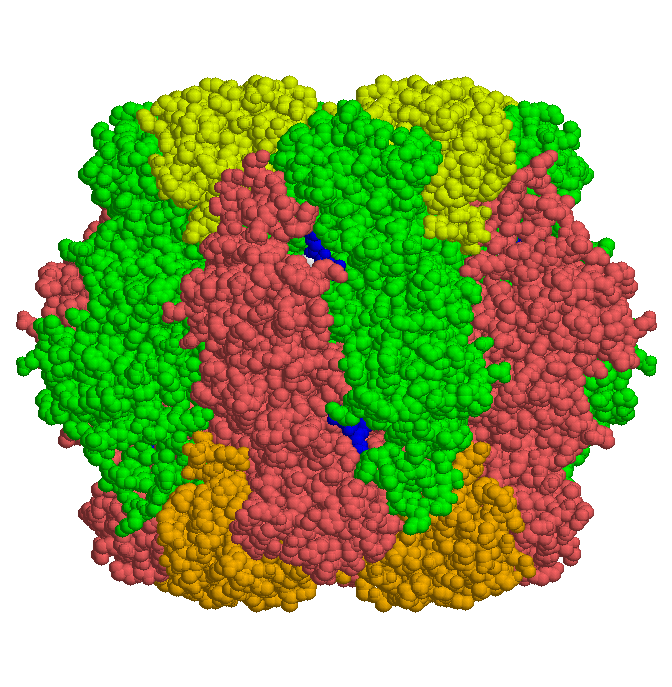 Some bacteria contain an enzyme consisting of two identical subunits. In higher organisms capable of photosynthesis a larger enzyme with eight small and large subunits each is found. The larger of the subunits is catalytically active.
Some bacteria contain an enzyme consisting of two identical subunits. In higher organisms capable of photosynthesis a larger enzyme with eight small and large subunits each is found. The larger of the subunits is catalytically active.
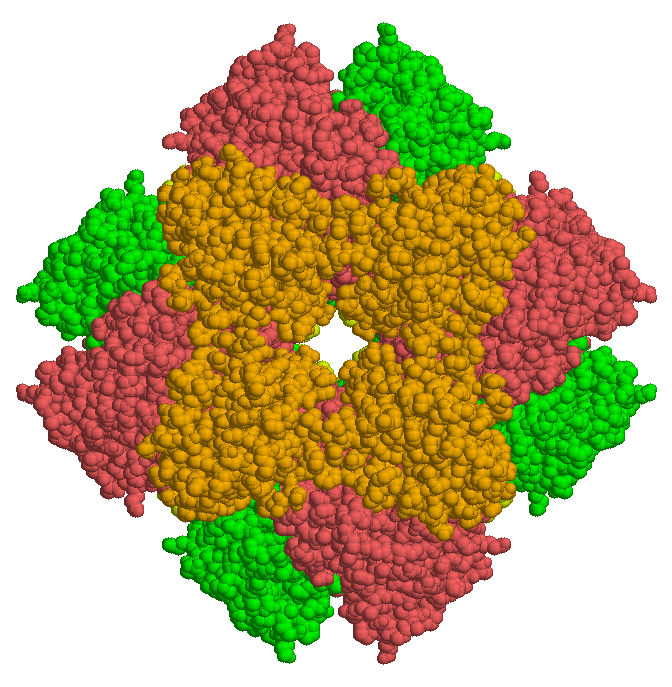
The structures of enzymes from Synechococcus, tobacco and spinach were elucidated. Here is shown the enzyme complex from spinach: large (dark) and small (light) subunits are organized in circles (red and green), which are stacked end-on symmetrically. To the right there is a side view, below is shown a view along the four-fold symmetry axis.
The enzyme from spinach was crystallized together with the substrate ribulose bisphosphate (blue in the above figure). The X-ray diffraction data of this complex gave clues to the reaction mechanism and it's stereo chemistry.
In the following scripts the structure of the enzyme as well as the reaction mechanism are demonstrated.