|
Q.
|
Rationale
|
|
1. A
|
Proteins
make up 50% of the dry weight of a cell.
|
|
2. B
|
Remember
CHNOPS!
|
|
3. D
|
The two
molecules shown are both amino acids -- they form a peptide bond.
|
|
4. B
|
Fatty
acids are the "monomers" for triglycerides, not hydrocarbons.
|
|
5. E
|
Rotate
molecule (a) upside down and it becomes (b); no isomers here!
|
|
6. B
|
Eukaryotes
are defined by the presence of a nucleus.
|
|
7. C
|
C-14 must
have two more heavy nuclear particles than C-12; if there were two extra
protons, this would no longer be Carbon but Oxygen.
|
|
8. E
|
Definition
|
|
9. E
|
Definition
|
|
10. B
|
Water
dissociates only weakly into H+ and OH-
|
|
11. A
|
pH is
a logarithmic scale; each downward step (e.g. 7 to 6) corresponds to
a tenfold increase in H+ ions.
|
|
12. D
|
This example
was discussed in class.
|
|
13. A
|
Structural
isomers because the carbonyl group is in a recognizably different position
(like moving one blue bead in an otherwise red chain from the end to
an interior position).
Note: the right figure in the exam included an unintended typo, an extra H atom attached to the keto group. This was corrected during the exam in the AS 55 classroom. Unfortunately, one of the proctors didn't show up, so I had to stay in AS 55 and couldn't go back to TLS 154 to announce the correction. |
|
14. A
|
Molecule
B is a triglyceride containing 3 fully saturated fatty acids.
|
|
15. D
|
Fats have
approximately 2x the energy content of sugars
|
|
16. D
|
Molecule
A is clearly a sugar; counting the carbons shows 5, a pentose.
|
|
17. A
|
Definition.
|
|
18. B
|
Example
discussed in class.
|
|
19. D
|
Definition
|
|
20. A
|
This is
a buffer. When OH- ions are added, more carbonic acid and bicarbonate
will dissociate to release H+, to combine with OH- and keep pH stable.
As this happens, the % of carbonate ion must increase.
|
|
21. A
|
Hydrolysis
is defined as the process of breaking covalent bonds by adding water.
|
|
22. C
|
Starch
is the common plant soluble polysaccharide, similar to animal glycogen.
|
|
23. A
|
Definition.
|
|
24. C
|
Enzymes
work without needing an external energy source; by rearranging electrons
they lower activation energies that prevent reactions from ocurring
rapidly.
|
|
25. E
|
DNA is
the information transmitting molecule, not proteins.
|
|
26. B
|
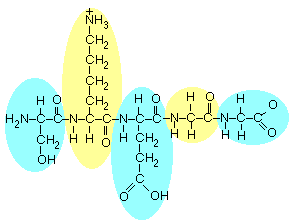
There
are 5 amino acids here, as indicated by circling them in the figure
below:
|
|
27. A
|
No -SH
groups are present.
|
|
28. A
|
Definition
|
|
29. C
|
Since
there is a hydroxyl group, this will be a polar side chain
|
|
30. B
|
Definition
|
|
31. B
|
Allosteric
enzymes are more complex and "expensive" for the cell to build
-- they typically occur only in the first step of a series of enzyme-mediated
synthetic reactions.
|
|
32. A
|
Disulfide
bonds are important in helping to maintain the stability of both 3o
and 4o structure.
|
|
33. A
|
The size
of a delta G tells you nothing about how fast the reaction will proceed.
|
|
34. A
|
This structure
shows membranes studded with particles, the "classic" appearance of
rER
|
|
35. A
|
Letter
"l" is pointing to the big densely stained body in the nucleus; this
must be the nucleolus.
|
|
36. E
|
All these
are membrane-bounded structures, and all membranes contain phospholipids.
|
|
37. D
|
-CH3 groups
are nonpolar, as are all hydrocarbons.
|
|
38. A
|
Definition
|
|
39. A
|
Any cell
that secretes protein must have lots of rER.
|
|
40. B
|
The nuclear
membrane is a double membrane.
|
|
41. A
|
Definition
|
|
42. C
|
Definition
|
|
43. C
|
(c) is
the only molecule that looks 'funny' - it doesn't appear to display
the correct valence for Carbon. The only way to account for this structure
is if there is a triple bond linking the two C atoms.
|
|
44. A
|
It's obviously
a fatty acid (hydrocarbon + carboxyl group), and since there's a double
bond it is by definition unsaturated.
|
|
45. C
|
Definition.
|