Bio 107 Fall 1999 Study Guide on Chs. 8 & 9
Ch. 8. Membrane Structure and Functions.
- What molecules compose cell membranes, and in approximately what %? What
is the difference between extrinsic (peripheral) and intrinsic (integral)
membrane proteins? What types of functions do membrane proteins carry out?
What function(s) do membrane lipids serve?
- What is the fluid mosaic model?
- Which of the following molecules could travel across a plasma membrane without
a protein carrier? water, glucose, oil, hemoglobin, sodium ion, messenger
RNA, ethanol.
- Which of the molecules listed in the previous question would require
a specific carrier in order to be transported into a cell? Is there anything
on the list that you would not expect to see transported across a membrane
under any circumstances? If so, what?
- Explain what is meant by the terms "isotonic", "hypertonic",
"hypotonic", "lysis", "plasmolysis", "shrinkage"
and "osmosis". What is the equivalent salt concentration of a human
cell?
- A wilted lettuce leaf is placed in fresh water. What happens? Which of the
terms mentioned above are involved?
- A cook pickles some tomatoes in concentrated brine. Fungi and bacteria (cells
with walls) can no longer grow. Explain. Which of the terms mentioned above
are involved?
- How does "facilitated diffusion" differ from "passive diffusion"?
How does "facilitated diffusion" differ from active transport? Which
of these processes would be affected by a drug that binds tightly to proteins
and blocks their activity?
- What is the difference between symport, antiport, and ATP pumps? What is
the immediate source of energy for each process?
- (a) Identify each of the following terms: phagocytosis, pinocytosis, receptor-mediated
endocytosis, exocytosis. Give an example of a situation in which each would
occur.
(b) explain the process by which iron gets from the bloodstream into human
cells. Compare this with the uptake of glucose.
- What function is served by gap junctions? desmosomes? tight junctions?
plasmodesmata? Which of these are found in animals? plants?
Ch. 9. Metabolism: Glycolysis, Fermentation, Respiration .
- Be able to recognize the structure of ATP, ADP, and AMP (see fig. 6.6).
Why is ATP so useful in cell metabolism?
- What are "redox reactions"? Why are they important in biology?
- Explain why removal of a hydrogen atom (H) is called an oxidation. Where’s
the electron?
- What is the difference between an electron carrier and a terminal
electron acceptor? Give examples of each.
- What does NAD+ do in biological systems? Relative to other biological
molecules, how much NAD+ is there in a cell? (sample answers: (a)
a lot; (b) comparable to the concentration of amino acids; (c) extremely little).
What happens to a cell when all its NAD+ becomes reduced to NADH?
- Be familiar with the process by which cells break down glucose sugar (glycolysis
followed by respiration). How much energy does this process yield aerobically?
anaerobically? How efficient are these two processes?
- Where does glycolysis occur? What are the end products? How many oxidation
reactions are involved?
- What does the TCA (Krebs) cycle accomplish? What is the starting material?
What are the final products?
- How is a mitochondrion organized? Be able to identify the matrix,
the cristae, and the intermembrane space in which H+
ions accumulate during proton gradient formation. What kinds of molecules
make up the electron transport chain? Where do electrons entering this chain
originate? Where do they end up?
- What is a proton gradient? How is it generated? Once made, how can
a cell use it to make ATP? What is the role of ATP synthase?
- Note that the term "chemiosmosis" refers to the coupling
of enzyme reactions to the generation of transmembrane proton gradient. Are
the terms "chemiosmotic phosphorylation" and "oxidative phosphorylation"
interchangeable?
- What is meant by the term "fermentation"? Identify two
organisms that can ferment. Identify two characteristic fermentation products.
Are these edible? Identify common food/beverage in which you would find each
of these two products.
- Contrast fermentation with respiration in each of the following respects:
(1) what happens to electrons in NADH? (2) how efficient is the process? (3)
where do the electrons made available in oxidation reactions wind up?
Additional practice questions (from prior exams)
- The delta Go’ of hydrolysis of (NADH + H+) is —54 kcal/mole
(a) How much ATP could theoretically be made from one mole of (NADH + H+)
(starting with ADP and Pi)?
(b) How much ATP is actually made from this (NADH + H+) in normal
respiration?
(c) How much ATP is actually made from this (NADH + H+) in fermentation?
- When electrons flow along the ETS chain of the inner membrane of the mitochondrion:
(a) The pH of the matrix decreases.
(b) The pH of the intermembrane space decreases
(c) Hydrogen ions are translocated into the matrix.
(d) ATP is synthesized directly by the redox reactions.
(e) Both b and d.
- The oxygen consumed during cellular respiration is directly involved in:
(a) Glycolysis.
(b) Accepting electrons at the terminus of the electron transport chain.
(c) The citric acid cycle.
(d) Oxidation of pyruvic acid to acetyl-CoA.
(e) Phosphorylation of ADP
- A jogger's muscles are metabolizing glucose faster than the muscle cells
can be supplied with oxygen. Under these anaerobic conditions, which substance
is not produced?
a. ATP
b. Pyruvic acid
c. Lactic acid
d. Acetyl-CoA
e. NADH
- For each O2 molecule you consume in cellular respiration, how
many CO2 molecules do you exhale?
(a) 1
(b) 12
(c) 6
(d) 3
(e) 36
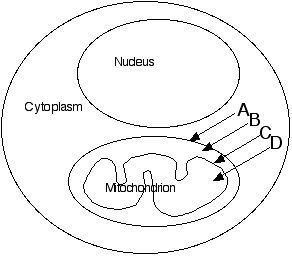
Questions 6—11 refer to the cell diagrammed above, which contains one
mitochondrion and one nucleus illustrated in very schematic form. Note that
some arrows point to COMPARTMENTS, while others point to MEMBRANES.
In which region(s) of the cell would you find: (Some questions may have
more than one answer.)
- Electron transport system (ETS) associated with chemiosmotic phosphorylation
- Enzymes of the glycolysis pathway
- ATP synthesis occurring
- Enzymes of the Krebs cycle
- H+ ions accumulating during electron flow
- Oxidation reactions occurring
