Cu centers from plastocyanin and cytochrome oxidase
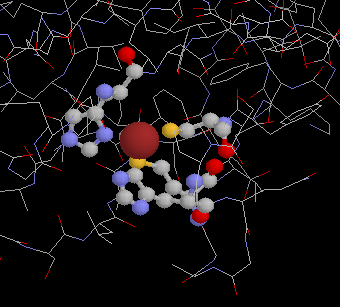
Cu center of plastocyanin
Half-cell reaction:
Cu2+ ==> Cu3+ + e-
- Midpoint redox potential: Em, pH 7 = 360 mV
- No. of electrons: z=1
- No. of protons: n=0 (n=1 below pK)
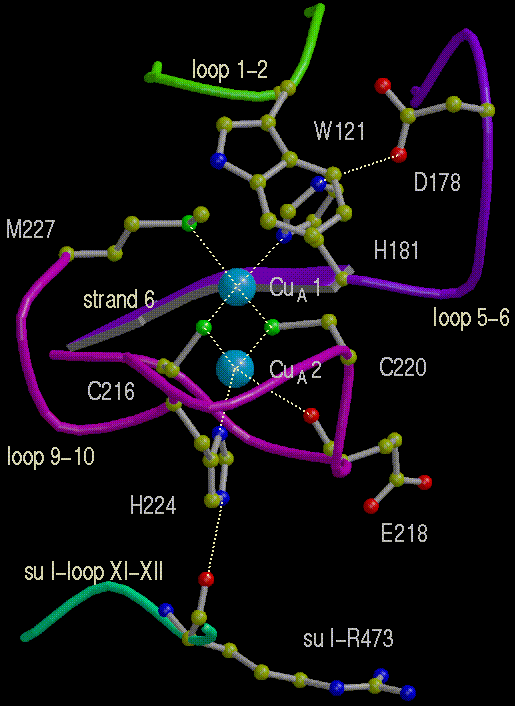
The two Cu center of CuA of cytochrome oxidase
Half-cell reaction:
CuA ==> CuA+ + e-
- Midpoint redox potential: Em, pH 7 = 280 mV
- No. of electrons: z=1
- No. of protons: n=0 or 1, depending on protein, pH, pK
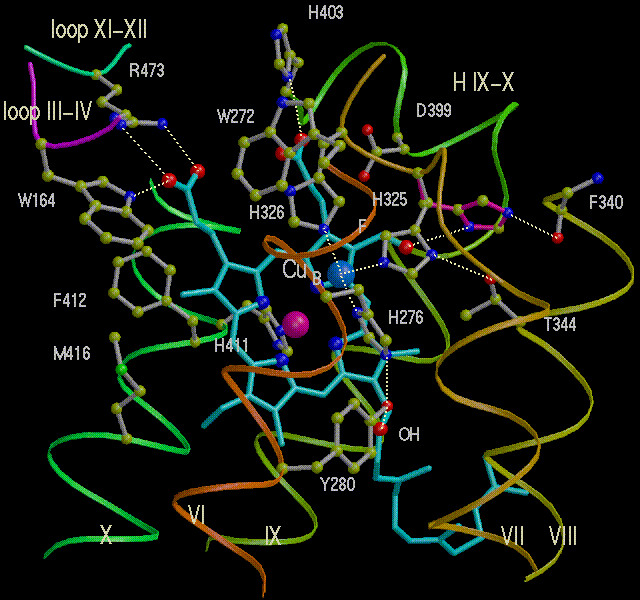
CuB at the binuclear center of cytochrome oxidase
Half-cell reaction:
CuB2+ ==> CuB3+ + e-
- Midpoint redox potential: Em, pH 7 = 340 mV
- No. of electrons: z=1
- No. of protons: n=0 or 1, depending on protein, pH, pK
©Copyright 1996,
Antony Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu


