Lecture 18Kinetics- simple rate equations |
The Arrhenius equation is based on the empirical observation that rates of reactions increase with temperature. This is accounted for by an increase in rate consant with temperature, which led to the concept of an activation barrier in the reaction pathway. The height of barrier is given by the activation energy, Eact.
where k¥ is the rate constant when Eact is zero, often called the pre-exponential factor. In collisional ractions, k¥ is the diffusion limited rate constant (see diffusion note above).
where rm and rn are the radii of reacting species (in cm), Dm and Dn are diffusion coefficients (units of cm2.s-1) , No is the Avogadro number, and the division by 1000 cm3 gives k in M-1 s-1.
Eyring, Randall-Wilkins theory:
Consider the reaction below, which proceeds through an activation barrier
populated by the species MN#:
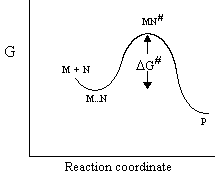
k1
k2
M + N <==> M--N <==> MN# ---->
P
k-1
The probability for forming MN#, the intermediate at the top of the activation barrier, is determined by the equilibrium constant for formation (K#), and hence the free-energy:
The rate of formation of products is given by:
The rate constant k2 is considered to occur in the first vibrational transition, and decay of MN# is assumed to go with equal probability forward or back, so that:
where kB is the Boltzmann constant, h is the Planck constant, and kBT/h has a value of ~1013 s-1. Substituting:
Since we can also express the rate in the conventional form:
and since K# = exp[-DGact/RT]
We introduce a second factor, k, to account for differences between adiabatic and non-adiabatic processes (see Lecture 19), and convert to molecular units by substituting kB for R, to get:
More generally, we can consider that the limiting step of a reaction may be the collisional process, in which case the overall rate will be determined by the rate of formation of M--N through the second order process above. Then:
where B is either collision frequency (the diffusion limited rate constant, also called A above, 1011 M-1.s-1) if liquid phase collisions are involved, or B is vibrational frequency (kBT / h (~1013 s-1)) if the reaction is intramolecular or monomolecular (first order), or some mixed term if a collisional process proceeds via an intramolecular complex.
If we equate Eact of the Arrhenius equation with DHact, then: