Model proton pump |
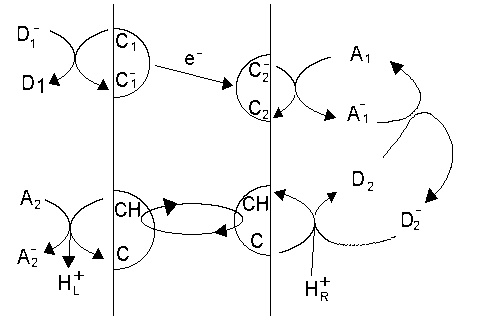
For the electrogenic arm
The work available to drive this is the redox potential difference between C1 and C2, or from the equilibria above, between D1 and A1:
At equilibrium, the two work terms balance, and (since the electron carries a negative charge):
For the neutral arm, with hydrogen carrier C.H+/CH, the oxidized and reduced forms of the couple can both freely diffuse in the membrane phase, and so have the same activity on both sides of the membrane. However, the potential of the couple will be different on the left and right, because the protons with which the couple equilibrates will be at a different activities in left and right aqueous phases. From the condition of equilibrium,
On the right,
On the left,
The pH gradient across the membrane will be maintained by the work term due to the difference in redox potential between couples A1 and A2, as shown by substitution between the equations above:
where Z = 2.303.RT/zF
Now, by substituting for E'A1 from eq. (1) in eq. (2), we can find the balancing work terms from the redox drop between D1 and A2, and from the proton gradient.
Note that, because the redox potentials of couples at equilibrium in any phase have the same value, we would have reached the same equation if C2 had donated electrons directly to the C/CH couple, or if the electron transfers between D1 and C1, and between CH and A2, had occured at the membrane/water interface, with D1 and A2 in the membrane rather than in the water. This latter set of conditions is appropriate for many of the proton pumps of electron transfer chains.