Redox chemistry of semiquinone systems |
The redox potential equations for these reactions can be written by substituting from the reaction equations into the standard equation. We will show only the equations for the protonated forms:
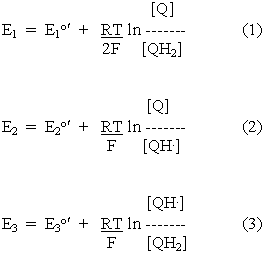
The equilibrium constant for this reaction is called the stability constant for semiquinone formation, since it determines the stability of the semiquinone form. Similar equations for the forms present at different pH values can be written, so that the terms in this equation can be taken to represent total semiquinone, total quinone and total quinol.
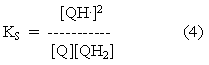
Two important equations can be obtained from these relationships, and from the condition that at equilibrium, E1 = E2 = E3 (since all three redox reactions are at the same ambient potential). Substitution from equations 2 and 3 into equation 1 gives:
Substitution from equations 2 and 3 into equation 4 gives:
An important consequence of these equations is the form of the titration curve for formation of the semiquinone as a function of redox potential. The curve is bell-shaped, with a maximal value at Eh corresponding to the Em value of the Q/QH2 couple from which the semiquinone is formed.