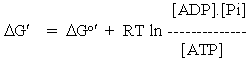Standard states and reference energy levels
Changes in energy content of a system are most easily compared if everyone agrees on a set of reference conditions. There are several conventions, but the commonly used set (which we will use in this course) refers changes in chemical systems to defined standard states. The primary reference is to:
The standard state of the elements, and the zero reference energy level
Elements in there standard states are considered to have chemical potentials and enthalpies of 0, or
µoelement = 0
The standard state of an element is its natural state at 1 atms pressure, 25oC.
By defining the free energy of the elements in this way, we can regard any compound as having a chemical potential (partial free enrgy), or an enthalpy of formation, composed of the sum of all changes in chemical potential (or of enthalpy) for the reactions leading to its formation, by any convenient path. Since free energy and enthalpy are variables of state, the value is a unique function of the state, so this approach can be used to define the the relative energy content of any chemical system by reference to the work needed to get there starting from the elements.
Standard states of solutes and gases, and free energy changes of reaction
In order to compare free energies for chemical processes, it is convenient to normalize free energy changes so as to eliminate differences in reaction volume. This is achieved by using 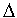 Go, the molar free energy change, and a standard state for reactants in solution.
Go, the molar free energy change, and a standard state for reactants in solution.
Under normal temperature and pressure (NTP) of 25oC and1 atms pressure:
Solutes are in their standard states when they have an activity of 1 M.
Gases are in their standard states when they are at a pressure of 1 atms.
Standard state of the solvent
Treatment of the solvent represents a tricky issue. By convention, the solvent is assumed to have a standard state of 1 M (the units being necessary to avoid dimensions in logaritmic terms), which does not change under the conditions of biochemical reaction (solutes at dilute solution). In the case of aqueous solutions, this may seem odd, because the activity of liquid water is 55 M (at NTP). This convention is adopted because the interactions with the solvent are subsumed under the chemical potentials of the solutes (which are referred to the standard state of a 1 M solution), and under the activity coefficients relating activities to concentrations. The convention allows one to ignore interactions between solute and solvent; this is usually appropriate since they are not significantly affected by changes in the solvent. In effect, for biochemical reactions in which water is a substrate or product (mainly hydrolysis and lyase reactions), water is ommitted from the thermodynamic equation. Thus for ATP hydrolysis:
ATP + H2O <===> ADP + Pi
©Copyright 1996,
Antony Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu