LESSONS FROM CLASSICAL THERMODYNAMICS
1) Define System as separate from Surroundings.
2) Identify Change in State that system undergoes (Initial and
Final states).
3) Change in state occurs through a process defined by a pathway.
Work (w) and heat (q) exchanged with the surroundings are dependent
on the pathway. However, we can in principle find a pathway for
which the change in state occurs (through appropriate coupling
to the surroundings), in which the maximal amount of work (wmax)
is done. Such a pathway defines a reversible process, and the
heat exchanged is called the reversible heat (qrev).
A reversible process does not occur at a finite rate.
FOR A REVERSIBLE PROCESS Wmax AND Qrev HAVE
FIXED VALUES.
Since we can in principle find a reversible process for any change
in state, we can also define the fixed values for maximal work
and reversible heat for any change in state.
The parameters, wmax and qrev, which define
the reversible process, are of special importance in thermodynamics,
because, unlike the work and heat exchanged in an irreversible
(or "spontaneous") process, they have unique values
for a defined change in state; they are variables of state.
Further analysis of the heat exchange led to the concept of entropy.
At constant T,
dE = wmax + qrev (for a "reversible"
process)
dE = dA + TdS (dS = qrev/T , Entropy change; TdS is
the reversible heat)
dA = dE - TdS (Helmholtz free energy change, or maximal work)
dG = dE - (-PdV) - TdS (Gibbs free energy change, or useful work)
dG = dH - TdS (dH = dE - (-PdV) , Enthalpy change)
dG = dA - (-PdV) (Useful work is maximal work minus the work done
against the atmosphere)
The Gibbs free energy differs from the Helmholtz free energy by
the work (-PdV) performed against the atmosphere, which is not
regarded as "useful". The Gibbs free energy is sometimes
called the "useful" work. For most biochemical processes,
no significant change in volume occurs, and dA and dG have the
same value.
5) Spontaneous process
During any process which occurs at a finite rate (a "spontaneous"
process), the system loses some capacity for performing work.
For all spontaneous processes, the change in state of the system
must have a negative value for dG. The process must (in principle)
be able to perform useful work on the surroundings, if a suitable
coupling process is available.
6) Change in state by a "spontaneous" process does not
occur by a reversible pathway. Work obtained is less than wmax
and the difference is passed to the surroundings as heat (entropy
increase of surroundings).
7) Conditions for spontaneous process
For System, change in state must have -dG
For System + Surroundings, entropy must increase (the entropy
of the universe is increasing to a maximum)
8) Because dE, dH, dG, dA, dS are variables of state, they have
fixed values for any defined change in state. From this it is
obvious that if a process occurs in which the system is returned
to its initial state, the values for changes in the variables
of state are zero (the cyclic integral for dG, dA, dH, etc. is
0).
9) Because dE, dH, dG, dA, dS, etc. are variables of state, any
defined change in state can be achieved by a series of changes
in state. The values for changes of each variable of state can
be summed along this series to give the overall value for the
change.
State 1 --------------------------------------------------------------->
State 2
State 1----> State a ----> State b ----> State c ---->
State d ----> State 2
dG(2-1) = dG(a-1) + dG(b-a) +
dG(c-b) + dG(d-e) + dG(2-d)
10) A change in state of the system can occur which involves many
different sorts of work (mechanical, pressure-volume, chemical,
electrical, electrochemical, etc.). In principle, we can imagine
a process in which each work term is performed separately (through
a separate change in state of the system), while all other variables
are held constant. Then the overall work will be the sum of the
separate work terms for the partial processes.
For a change in state of the system which involves exchange of
heat, pressure-volume work, mechanical work, and chemical work,
we may write an equation for the change in free energy
dG = -SdT + (-VdP) + fdl + µidni
Each of the work terms here is the product of an intensity factor
(the driving force) and a capacity factor (dependent on the size
of the system). The intensity factor for chemical work is the
chemical potential (see below, and the other pages).
CHEMICAL THERMODYNAMICS
1) Chemical potential
The chemical potential of a system is defined by the change in free-energy of the system as a function of the change in composition. For a chemical species, i,
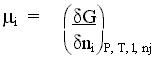
the chemical potential of i is the rate of change of free energy with change in the number of moles of i , when all other components, and work terms, are held constant.
2) Reference states
In considering chemical thermodynamics, we must talk about changes
in state of the system. The system is defined by a chemical mixture
of reactants and products, the change in state is a change in
concentrations of the reactants as the process proceeds, and the
process is defined by a chemical equation. By convention, a chemical
equation is written with reactants on the left, and products on
the right, and the process is assumed to occur from left to right.
It is convenient to agree on states of chemical systems to which
we an refer changes. These states are:
Zero chemical potential. Complex chemicals are synthesized
from the elements. The elements are assumed to have a chemical
potential of zero in their natural states.
Standard states. For chemical or biochemical species in
solution, the standard state is a solution of activity 1 molar
(1 M). For gases, the standard state is the gas at 1 atmosphere
pressure. For solvents, the activity in the pure state is arbitrarily
taken as 1. This is particularly important in biochemical systems,
where the aqueous solvent is assumed to have an activity of 1,
which does not change significantly during a reaction. The temperature
of reference states is assumed to be 25o C unless otherwise
indicated.
Standard chemical potential and chemical potential. The
standard chemical potential of a species i (µoi
) is its chemical potential in the standard state (an activity
of 1 molar). The chemical potential at any other activity is given
by:
µi = µoi + RT ln (ai
/ aio)
where aio is the activity in the standard
state. Since the value for aio is by definition
1 M, its inclusion in the above equation makes no difference to
the numerical value of the logarithmic term. Inclusion does make
the term in parentheses a dimensionless ratio, and this is important
in understanding what's going on algebraically. However, the convention
is to ignore this term, and to write the above equation as:
µi = µoi + RT ln (ai)