|
|
Quiz (Ch. 33) Quiz (Ch. 29) |
Antimicrobial Chemotherapy. Nonspecific Host Defenses |
|
|
|
Lecture Index | ||
|
|
Course Resources page |
Last revised: Friday, April 21, 2000
Ch. 33; Ch. 20 (p. 591-602) in Prescott et al, Microbiology, 4th Ed.Note: These notes are provided as a guide to topics the instructor hopes to cover during lecture. Actual coverage will always differ somewhat from what is printed here. These notes are not a substitute for the actual lecture!Copyright 2000. Thomas M. Terry
Antimicrobial Drugs
So far, talked about agents applied outside body. Rise of chemotherapeutics was major revolution in medicineA. History:
- Antibiotics known for long time= chemicals produced by certain organisms that killed other organism. E.g. mushroom poisons. Early searches for antibiotics (ca. 1900) had bad side-effects. People decided that therapeutic applications were probably too dangerous.
- Ehrlich's "magic bullet": 1909, discovered "Salvarsan", chemical used to treat syphillis. Ehrlich stressed Selective toxicity as key factor in success.
B. Structural analogues as drugs
- 1935. Domagk discovered sulfa drugs.
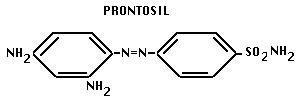
- this drug prevented Staph aureus infections in vivo, but not on petri plates. Domagk discovered that compound is split in liver into two parts, including:
- Bacteria normally synthesize folic acide (cofactor for synthesis of bases for DNA and RNA) from PABA:
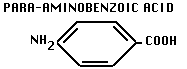
- But sulfanilamide is competitive inhibitor, blocks folic acid synthesis, blocks NA production.
- Humans can't make folic acid (vitamin requirement), hence are not affected.
- optimal use: E. coli urinary tract infections, certain protozoal infections, Nocardia infections. Not good if pus or dead tissue involved.
- Sulfa drugs had enormous impact on WWII. First battles in which more men died in battle than afterwards as result of infection.
- Note: later modified: sulfanilamide is insoluble in acidid urine, causes kidney problems. Chemical modification can produce drug that has same activity, but is more soluble in urine:
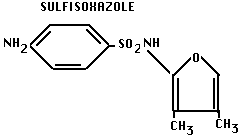
C. Antibiotics:
- After Sulfa drug success, more search for drugs.
- Penicillin was discovered in 1929, not commercially developed until WWII.
- Since then major search for antibiotics. Found in 3 major groups of microorganisms:
- Certain molds (Penicillium, Cephalosporium). e.g. penicillin,cephalosporin
- Certain strains of Bacillus e.g. bacitracin
- Many strains of Actinomycetes (soil bacteria that grow in long filamentous masses). Especially from Genus Streptomycetes. e.g. streptomycin. Majority of antibiotics come from these organisms.
1. Cell Wall antibiotics
- Penicillins. First widely available drug, introduced in 1945. Contains ß-lactam ring. Benzylpenicillin (Penicillin G) was first natural isolate
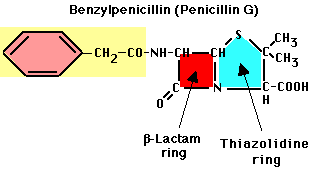
- Activity: binds to enzymes that carry out transpeptidation linkage in bacterial cells. Unique target in procaryotes.
- Note beta-lactam ring -- this is critical for activity.
- INITIAL PRODUCTION: 1-10 ug/ ml. Gradual strain improvement over years, today 85,000 ug/ml!!!
- BenzylPenicillin G -- low activity vs Gram-, ß-lactamase sensitive
- Modifications: side chain can be chemically modified. e.g.
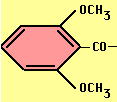
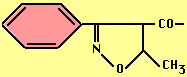
- Methicillin, Oxacillin -- acid stable, ß-lactamase resistant
Methicillin
Oxacillin
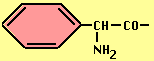
- Ampicillin -- broader spectrum (esp. vs Gram-), acid stable, ß-lactamase sensitive.
- Carbenicillin -- broader spectrum (esp. vs Pseudomonads), acid stable but not effective orally, ß-lactamase sensitive
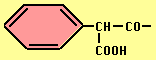
- Other antibiotics active against growth of cell wall: Cephalosporins, Cycloserine, Bacitracin.
- Cephalosporins (e.g. cefoxitin, cephalothin) are ß-lactam antibiotics, dihydrothiazine ring instead of thiazolidine ring. Broader spectrum than penicillins, low toxicity. Relatively resistant to pencillinase.
- Bacitracin does not block transpeptidation, but previous step in wall synthesis. Limited to topical application, because of severe toxic reactions.
2. Inhibitors of protein synthesis
- Aminoglycosides: (have amino sugars linked by glycosidic bonds). streptomycin, gentamicin, kanamycin, tobramycin, amikacin. Used mainly for Gram- infections. Not very effective against anaerobes or Gram+. Bind to 30s ribosomal unit, block protein synthesis. Also cause misreading of mRNA. Useful for a number of diseases. Different degrees of toxicity (e.g. Gentamicin is very toxic, only used for severe infections) .
Amikacin is best antibiotic for Gram- rod hospital infections, because such infections (nosocomial) are often caused by strains with R-factors, resistant to many common antibiotics. Generally group is used as reserve antibiotics, when others fail. - Tetracyclines. (4 ring system) Also bind to 30S subunit,block protein synthesis. Effective against variety of pathogenic bacteria- broad spectrum. Together with ß-lactam antibiotics, the most important group commercially.
- Glycopeptide antibiotics. E.g. Vancomycin.
- Two antibiotics commercially available: vancomycin & teicoplanin.
- Vancomycin (Vancocin) -- worldwide use. Discovered 1956, produced by fungus Amycolatopsis orientalis, from Indonesia. Drug has relatively high toxicity and requires IV administration. Currently the drug of last resort for treating methicillin-resistant staph infections.
- Teicoplanin (Targocid) -- another glycopeptide, approved for use in some European countries in 1988, under clinical trials in U.S. and Canada. Has longer half-life than vancomycin, can be administered only once a day by intramuscular or IV. Is effective against some vancomycin-resistant strains.
- Macrolide antibiotics. E.g. Erythromycin. Large lactone rings connected to sugar groups. Binds to 50S ribosome, blocks protein synthesis. Most active vs. Gram+ organisms, eg. Strep. pyogenes. Now routinely applied to eyes of newborns to prevent gonnorhaea and chlamydia from infecting eye.
D. Drug resistance
Testing for Drug Resistance
- Plate sensitivity test: (will be done in MCB 229 lab)
- add test bacteria to small amount of melted agar
- pour over surface of nutrient agar plate, let gel
- add paper disks with known dose of antibiotic to surface
- incubate: antibiotic will diffuse into medium as cells grow
- examine plate: look for clear zones around disk where growth is inhibited
- measure diameter of clear zones: consult table to find if this is clinically useful
History:
- Drug resistance first noted in Japanese hospitals; serious increase in bacterial strains resistant to variety of standard antibiotics.
- Since then, many examples of drug resistance developing. Ex: gonorrhea initially treated by pencillin. But pencillin-resistant strains now account for more than 25% of isolates, must use different antibiotic.
- Note: antibiotic resistance has always been present; frozen bacterial cultures from before WW II have been shown to include drug resistant individuals even though antibiotics weren't yet used by humans. Conclude that antibiotics are natural part of biological activity, not surprising that some resistance should have developed in course of evolution.
- What is new, and different, is rate of development of resistance. Some disesase, like TB, never easy to treat even with the few antibiotics that were effective. Now drug resistant strains appearing, TB becoming much harder to treat.
Different ways for bacteria to develop drug resistance
- mutations affecting cell surface can affect entry of drug
- prevents entry of drug into cell
- receptor normally used by drug altered- no binding.
- example: mutations can affect drug target in cell (e.g. slight change in ribosomal RNA can change affinity of ribosomes for erythromycin)
- bacteria or plasmids can produce enzymes which inactivate drug; e.g. pencillinases hydrolyze ß-lactam ring.
- Plasmids = small, circular DNA elements that reside in bacterial cells, duplicate separately from bacterial chromosome.
- Some plasmids carry genes for antibiotic resistance (enzymes that degrade antibiotic). Called R-plasmids. Have been found for most classes of antibiotics.
- When antibiotics are in use, most bacteria are killed. If R-plasmid exists, can be transferred to other cells, resistance spreads through population. Result: new population is resistant to drug.
- Note: possible for a single plasmid to carry multiple drug resistance genes, spread all of these as a single unit!
- plasmid encoded drug pump
- production of protein "pumps" to pump drug out of cell
Ways to deal with antibiotic resistance
- higher dose, different antibiotic, more than one drug simultaneously
- also restraint by physicians and control (no over the counter use)
- CORRECT use of drug. Most people take drugs improperly, miss doses, allow conditions that favor selection of drug resistant mutants.
A. Non-specific Defenses
Tissue barriers
- Skin & Mucous membranes
- Respiratory tract
- Intestinal tract
- Genitourinary tract
Chemical barriers
- Include a variety of enzymes; lysozyme, fibronectin, hormones (such as corticosteroids), etc.
Phagocytes
- Blood consists of both red blood cells (carry O2 and CO2) and white cells (major components of immune system). View picture of blood cells.
- Certain white blood cells (granulocytes, macrophages, others) are highly mobile, carry out phagocytic ("cell eating") activity
- White blood cells are chemically attracted to foci of disease or tissue damage by process of chemotaxis = chemical signals stimulate motion towards the attractant
- View movie demonstrating chemotaxis (from Cells Alive, by James A. Sullivan)
- Phagocytosis begins with engulfment of particulate matter (can be bacteria, clumps of virions, cell debris, etc.) into phagosome.
- View images and movie demonstrating phagocytosis (from Cells Alive, by James A. Sullivan)
- Phagosome fuses with lysosomes ---> phagolysosome. Two kinds of lysosomes:
- acid hydrolases, lysozyme, neutral proteases, myeloperoxidase;
- lactoferrin, lysozyme, phospholipase A.
- These enzymes can degrade biomolecules; but many pathogenic bacteria have walls that are resistant to lysozyme.
- View animation of stages of phagocytosis
- Another feature of phagocytes: "Respiratory burst" (not associated with energy production in mitochondria). During phagocytosis, see dramatic increase in oxygen uptake. Produces superoxide (O2-), hydrogen peroxide (H2O2).
- View respiratory burst
- Note: certain people genetically lack capacity for respiratory burst; they cannot kill many pathogens that ordinary people can, are at much greater risk from infections.
Inflammation
- A nonspecific reaction to tissue damage. Stimulated by complex series of steps, initiated by cell damage.
- Effects include:
- vasodilation (opening junctions between capillary cells, allowing fluid and WBCs to leave blood and enter surrounding tissues) ---> swelling of afflicted tissues
- redness (from heightened blood flow)
- pain (from prostaglandins released by tissues binding to nerve receptors)
- heat (produced by pyrogens liberated at site of inflammation); may inhibit microbial growth
- a variety of altered functions at site of inflammation; fibrin clotting, platelet aggregation, chemotactic signaling to attract WBCs, activation of complement factor C3.
- View diagram summarizing effects of inflammation (scroll down the page to the first diagram).
Take a Self-Quiz on Ch. 33 material
Take a Self-Quiz on Ch. 29 material
Return to Lecture Index
Return to MCB 229 Course Resources page