Hantaviruses have been implicated as etiologic agents for two acute diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Both diseases are carried by rodent vectors. HFRS viruses are carried by Old World rodents, and HPS viruses by New World rodents. There are currently at least 6 hantaviruses indigenous to Old World rodents (including 3 that cause HFRS) and at least 8 among indigenous American rodents (including 4 that cause HPS). The true number and disease potentials of hantaviruses is doubtlessly much higher than currently appreciated. Hantaviruses are classic "emerging viruses" because of their tendency to appear, sometimes explosively, in new populations in which they are unexpected.
Hantaviruses are serologically-related members of the family Bunyaviridae (Elliott, 1991).


electron micrographs of negatively-stained hantavirus particles (CDC)
They are enveloped viruses with a tripartite negative-sense RNA genome. The three genome segments are called L, M and S; they encode the viral transcriptase, envelope glycoproteins, and nucleocapsid protein, respectively.
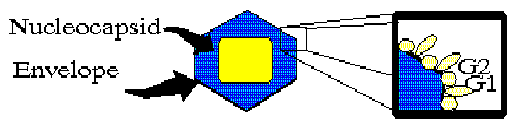
diagram showing genome and basic virion structure and coding assignments (B. Hjelle)
The prototype hantavirus is Hantaan virus (HTN), which infects the Asian striped field mouse Apodemus agrarius (Lee, 1978, Yanagihara, 1987a). In the rodent host HTN causes a chronic infection that does not appear to result in disease. When the virus is transmitted from mice to humans, an acute disease known as hemorrhagic fever with renal syndrome (HFRS, Korean hemorrhagic fever) results. Similar syndromes, although generally less severe, are caused by the related Puumala virus (PUU), Seoul virus (SEO), and Belgrade (Dobrava, DOB) virus. Mortality rates for HTN and DOB HFRS are the highest among the hantavirus- associated nephropathies, at approximately 5-20%. It is estimated that more than 100,000 people in China contract HFRS annually (Yanagihara, 1987a). All of the hosts for viruses associated with HFRS are associated with rodent hosts indigenous to the Old World (see Table 1). SEO is somewhat exceptional in that the worldwide spread of its host rodent, Rattus norvegicus, has resulted in the worldwide spread of SEO.
HFRS is an acute febrile illness that was first recognized in the western world after a 1951 outbreak among U.S. troops stationed in Korea. Fever and myalgia (muscle pain) develops days or weeks after exposure to rodents. This is followed by flushing, then petechiae. The disease progresses to hemorrhage (gastrointestinal, subconjunctival) and hemodynamic instability, occasionally progressing to shock. Thrombocytopenia, neutrophilia with left shift, atypical lymphocytes and hemoconcentration are common. There is acute renal failure, but the precise pathophysiologic lesion producing renal failure is uncertain. Mortality is usually from shock or hemorrhage. Pulmonary edema is a frequent finding (Powell, 1954).
The prototypic New World hantavirus is Prospect Hill virus (PH) of the meadow vole Microtus pennsylvanicus. It has not been associated with human disease, but is widespread among voles in the United States and Canada (Lee, 1982; Yanagihara, 1987b). Serologic evidence for hantavirus infection in deer mice (Peromyscus maniculatus) has been recognized for several years (Tsai, 1985, Yanagihara, 1987b; Yanagihara, 1990) but was generally ascribed to "spillover" of PH from M. pennsylvanicus. Isolation attempts had been unsuccessful.
Despite extensive study, no arthropod vector has been implicated in the transmission of any hantavirus. The route by which the virus is transmitted to humans therefore is believed to be via aerosols of infected excreta of rodents (Zu, 1985; Tsai, 1987). Infected rodents appear to be persistently infected and viruric. HTN seroprevalence among mice in endemic areas, as assessed by seroreactivity to culture-adapted Hantaan virus, fluctuates in a biphasic manner. A small peak of seroprevalence in the spring season usually is followed by a decline over the summer months; then a higher peak occurs in the autumn. The incidence of HFRS among humans follows a similar trend, but the curve slightly trails the seroprevalence curve among A. agrarius populations. Those individuals whose occupations (eg, farmer) place them at greater exposure to dust or aerosols containing A. agrarius excreta are the most commonly affected.
HPS was first recognized on May 14, 1993, when the New Mexico Office of the Medical Investigator notified the New Mexico Department of Health of a cluster of 3 unexplained pulmonary deaths occurring in the Four Corners region of the United States. “Four Corners” is so named because it is the only place in the United States in which four states share a common border: Utah, Arizona, New Mexico, and Colorado. On May 17, 1993, Dr. Bruce Tempest of the Indian Health Service in Gallup, New Mexico, independently noted a cluster of 5 deaths occurring in Four Corners, and notified health authorities.
A conference was assembled among local physicians and state and federal health authorities, even as additional cases began to be recognized. The common clinical features among the case-patients included a prodromal illness of fever, chills, and myalgia. The prodrome was followed by dyspnea, cough, thrombocytopenia, severe hemodynamic instability, neutrophilia with immature forms, atypical lymphocytes, elevated serum levels of lactate dehydrogenase. There was a high mortality rate, approximately 80 % in the initial group of patients. The chest X-ray examinations revealed a diffuse, interstitial infiltrate that resembled that observed in patients with adult respiratory distress syndrome (ARDS), which is a common pattern in patients who are extremely ill from any of a variety of diseases (e.g., bacterial sepsis or trauma). The new disease was thus called unexplained ARDS (UARDS; Duchin, 1994; Nolte, 1995).
Although UARDS did not feature significant renal disease, its overall similarity to HFRS led to the inclusion of “hemorrhagic fever viruses” on the short list of possible etiologies, along with a previously unknown “new virus”. This concern led to the examination of serum samples from patients with UARDS for antibodies to known agents of hemorrhagic fever. Some UARDS patients, it was discovered, had antibodies that showed reactivity to known hantaviruses. The pattern of reactivity, which was low titer but crossreactive with several hantaviruses, suggested that UARDS was caused by a novel hantavirus. When a polymerase chain reaction assay was developed that allowed the amplification of a previously- undescribed hantavirus from the autopsied tissues of patients with UARDS, the disease was renamed HPS (Nichol, 1993). Rodent trapping at homesites of HPS patients in Four Corners led to the recognition that the deer mouse (Peromyscus maniculatus)

is the predominant host for the Four Corners virus (Childs, 1994). This is a highly successful rodent species, with a propensity to entire human dwellings to obtain food. The virus has been isolated from deer mice in California (Schmaljohn, 1995) and New Mexico (Elliott, 1994).
FC is genetically (Nichol, 1993; Hjelle, 1994a; Spiropoulou, 1994) and serologically (Jenison, 1994) distinct from all previously-characterized hantaviruses. At the time of its recognition, it had closest resemblance to PH and PUU. Since that time, other hantaviruses that are more closely similar to FC have been discovered. Figure 1 is a phylogenetic tree depicting the genetic interrelationships among members of the hantavirus genus.
As of mid-January, 1995, HPS has been recognized 102 patients in 21 states of the U.S., as well as 7 in Canada and 3 in Brazil. The overall mortality is now approximately 40%; the decline can probably be attributed to improved fluid management in severely ill patients, and recognition of more patients with mild disease. There remains no specific treatment, although ribavirin was used in an uncontrolled trial without obvious success. Although the highest caseload of HPS still occurs in the Four Corners states, it appears that all states within the range of P. maniculatus are susceptible to HPS. Genetic and serologic investigations have shown that the majority, if not all, of the case-patients who have developed HPS in the western United States have been infected with the Four Corners virus (FC; CDC, 1994a; Hjelle, 1994b, c, d).
Several cases that have occurred outside of the range of P. maniculatus have shown that hantaviruses other than FC can induce HPS. Viral cDNAs with distinctive genetic features have been amplified from the tissues of case- patients in Brazil and Louisiana (Morzunov, 1995). The rodent hosts for those hantaviruses are not known. Another patient died of HPS in Rhode Island in January 1994. His recent travel history included numerous sites in New York (including Shelter Island) and Rhode Island, but did not include any region within the range of P. maniculatus (Brackett, 1994). In addition, serologic features of his virus were distinct from those of FC, and its genetic material proved to be unlike that of FC (Hjelle, 1995b). White-footed mice (Peromyscus leucopus) were captured on Shelter Island by E. Mackow in a search for the rodent carrier. This work resulted in the isolation of a novel hantavirus (NY-1) from a seropositive P. leucopus that bore striking genetic similarity to the virus from the Rhode Island case-patient (Song, 1994). These studies showed that the infection was most likely contracted on or near Shelter Island, and that the white-footed mouse (host to babesiosis and Lyme disease) is another host for HPS-associated viruses in the United States.
A nonfatal case of HPS occurred in Dade County, Florida, in late 1993. Although the virus was cleared from the blood of the case-patient before it could be subjected to molecular analysis, its serologic features suggested close relationship to FC (CDC, 1994b; Hjelle, 1994c). Trapping of rodents at the likely site of exposure showed that only cotton rats (Sigmodon hispidus) could be identified as hantavirus carriers. Serum antibodies from the case- patient reacted more strongly to antibodies from the cotton rat hantavirus (Black Creek Canal virus, BCC) than to antigens derived from FC (CDC, 1994c).
Three different genetic lineages of hantaviruses occur in three separate subfamilies of rodents within the family Muridae. A. agrarius and Rattus norvegicus are members of the subfamily Murinae, family Muridae. The majority of hantavirus isolates have come from these rodents. A second subfamily of murid rodents is Arvicolinae. This subfamily includes the genera Microtus and Clethrionomys. The former genus includes hosts for PH, Tula virus (TUL), Isla Vista virus (IV), and Bloodland Lake virus (BL), Clethrionomys is host to PUU. Of this group, only PUU has been linked to human disease (HFRS; Lee, 1982; Plyusnin, 1994; Song, 1995; Brummer- Korvenkontio, 1980). The subfamily Sigmodontinae includes deer mice, white-footed mice, cotton rats, and harvest mice. Members of this subfamily are hosts to FC, El Moro Canyon hantavirus (EMC), Rio Segundo hantavirus (RS), BCC, and NY-1. To date, all hantaviruses that have been implicated in HPS are either proven or suspected to be associated with rodents of the subfamily Sigmodontinae, family Muridae.
The serologic and genetic relationships among the various hantaviruses parallels the genetic relationships among the predominant rodent hosts for each virus (Lee, 1985; Chu, 1994; Xiao, 1994; Hjelle, 1994e, 1995d; reviewed in Hjelle, 1995c). The close parallel between the genetic interrelationships of hantaviruses and those of their rodent hosts argues that hantaviruses did not emerge through recent mutation or segment reassortment, but instead through ecological disturbances that bring hantavirus-infected rodents into closer contact with man. In fact, the data argue that hantaviruses have an ancient association with their hosts, perhaps even coevolving and cospeciating with their predominant rodent carriers. The oldest case of hantavirus pulmonary syndrome known to date occurred in 1959. Using a serologic diagnostic test (nucleocapsid and G1 antigen western blot), it has been shown that this case-patient (and others occurring in 1975 and 1985) had an antibody response that strongly indicates that FC, and not another hantavirus, was responsible for this case of HPS. In fact, genetic sequence studies have demonstrated that a virus that is indistinguishable from FC was present in deer mice in Mono County, California in 1983 (Nerurkar, 1993, 1994), and Kern County, California in 1975 (Sesline D and Hjelle B, unpublished data, 1994). The emergence of HPS in the southwestern United States has been linked to a well-documented rodent population boom in the region of fall, 1992 (Parmenter, 1993).
Early diagnosis of patients with HPS utilized culture-adapted SEO, PUU, PH, and HTN hantaviruses as antigen to detect FC antibodies (Nichol 1993; Childs, 1994). These heterologous antigens were recognized at the time to be suboptimal in detection of FC antibodies. RT-PCR was sensitive in detection of FC RNA both in patients who came to autopsy (Nichol, 1993) and in the peripheral blood mononuclear cells of living patients (Hjelle, 1994d, 1995a). Furthermore, viral antigens could be detected in tissue sections by immunostaining, using a monoclonal antibody directed against PUU (Zaki, 1994). To develop rapid diagnostic capabilities for FC antibodies, investigators at University of New Mexico Health Sciences Center (UNM HSC) and Centers for Disease Control and Prevention (CDC) independently developed antigens derived from FC, using recombinant DNA methodology. CDC developed an enzyme-linked immunosorbent assay (ELISA) based upon expression of recombinant FC nucleocapsid protein, while UNM HSC investigators developed a western blot assay for the FC nucleocapsid protein and the FC G1 glycoprotein (Feldman, 1993; Jenison, 1994; Yamada, 1995; Hjelle, 1994b,c).
While both systems provide rapid and sensitive diagnosis for serum antibodies directed against FC, the G1 glycoprotein antigen provided an additional advantage by allowing for specific diagnosis of FC infection. In contrast to the nucleocapsid antigen, which is broadly crossreactive among numerous hantaviruses, antibodies to the G1 glycoprotein of FC do not recognize the G1 glycoprotein of other hantaviruses such as PH or PUU (Jenison, 1994; Jenison, 1995). Despite this high specificity, there is a high degree of conservation of the G1 glycoproteins among different FC isolates detected throughout the western U.S. (Hjelle, 1994c). Both the CDC ELISA and UNM HSC western blot have been used extensively in the diagnosis of hantavirus infection in humans and rodents in the Americas.