It can be quite correctly argued that life exists on Earth because of the abundant liquid water. Other planets have water, but they either have it as a gas (Venus) or ice (Mars). The chemical nature of water is thus one we must examine as it permeates living systems: water is a universal solvent, and can be too much of a good thing for some cells to deal with.
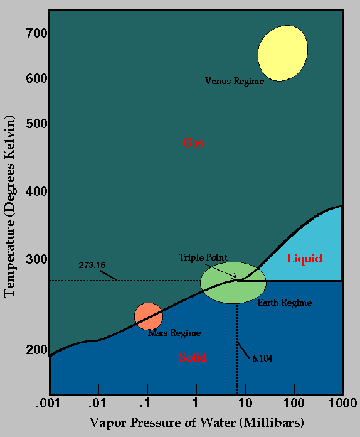
The above graph is from http://www.crseo.ucsb.edu/IOM2/Triple_Point.html. Note that water can exist in all three states of matter on Earth.
Water is polar covalently bonded within the molecule. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Other molecules, such as Ethane, are nonpolar, having neither a positive nor a negative side.
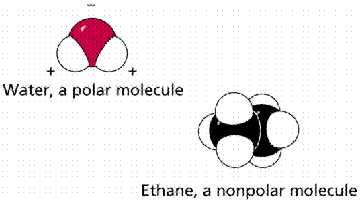
The difference between a polar (water) and nonpolar (ethane) molecule is due to the unequal sharing of electrons within the polar molecule. Nonpolar molecules have electrons equally shared within their covalent bonds.
These link up by the hydrogen bond discussed earlier. Consequently, water has a great interconnectivity of individual molecules, which is caused by the individually weak hydrogen bonds that can be quite strong when taken by the billions.
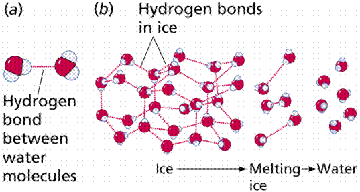
Formation of a hydrogen bond between the hydrogen side of one water molecule and the oxygen side of another water molecule.
Water has been referred to as the universal solvent. Living things are composed of atoms and molecules within aqueous solutions (solutions that have materials dissolved in water). Solutions are uniform mixtures of the molecules of two or more substances. The solvent is usually the substance present in the greatest amount (and is usually also a liquid). The substances of lesser amounts are the solutes.
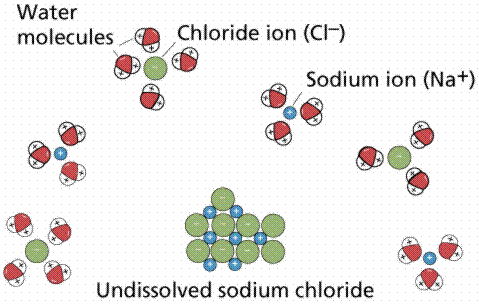
Dissolution of an ionically bonded compound, sodium chloride, by water molecules.
The solubility of many molecules is determined by their molecular structure. You are familiar with the phrase "mixing like oil and water." The biochemical basis for this phrase is that the organic macromolecules known as lipids (of which fats are an important, although often troublesome, group) have areas that lack polar covalent bonds. The polar covalently bonded water molecules act to exclude nonpolar molecules, causing the fats to clump together. The structure of many molecules can greatly influence their solubility. Sugars, such as glucose, have many hydroxyl (OH) groups, which tend to increase the solubility of the molecule.
Water tends to disassociate into H+ and OH- ions. In this disassociation, the oxygen retains the electrons and only one of the hydrogens, becoming a negatively charged ion known as hydroxide. Pure water has the same number (or concentration) of H+ as OH- ions. Acidic solutions have more H+ ions than OH- ions. Basic solutions have the opposite. An acid causes an increase in the numbers of H+ ions and a base causes an increase in the numbers of OH- ions.
The above slides are from James K. Hardys's site at the University of Akron.
The pH scale is a logarithmic scale representing the concentration of H+ ions in a solution. Remember that as the H+ concentration increases the OH- concentration decreases and vice versa . If we have a solution with one in every ten molecules being H+, we refer to the concentration of H+ ions as 1/10. Remember from algebra that we can write a fraction as a negative exponent, thus 1/10 becomes 10-1. Conversely 1/100 becomes 10-2 , 1/1000 becomes 10-3, etc. Logarithms are exponents to which a number (usually 10) has been raised. For example log 10 (pronounced "the log of 10") = 1 since 10 may be written as 101. The log 1/10 (or 10-1) = -1. pH, a measure of the concentration of H+ ions, is the negative log of the H+ ion concentration. If the pH of water is 7, then the concentration of H+ ions is 10-7, or 1/10,000,000. In the case of strong acids, such as HCl, an acid secreted by the lining of your stomach, [H+] (the concentration of H+ ions, written in a chemical shorthand) is 10-1; therefore the pH is 1.
Organic molecules are those that: 1) formed by the actions of living things; and/or 2) have a carbon backbone. Methane (CH4) is an example of this. If we remove the H from one of the methane units below, and begin linking them up, while removing other H units, we begin to form an organic molecule. (NOTE: Not all methane is organically derived, methane is a major component of the atmosphere of Jupiter). The resultant molecule is Butane, which has a chemical formula C4H10. Molecules made up of H and C are known as hydrocarbons.
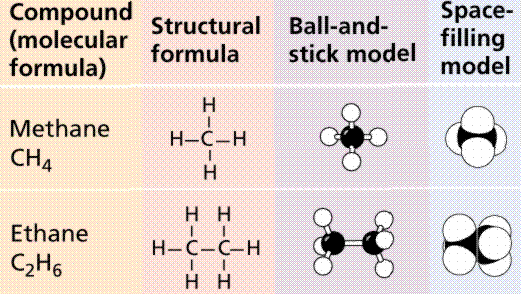
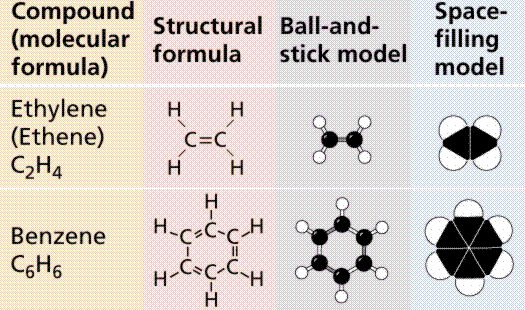
Types of hydrocarbon compounds.
Scientists eventually realized that specific chemical properties were a result of the presence of particular functional groups. One of the most common groups is the -OH (hydroxyl) group. Its presence will enable a molecule to be water soluble.
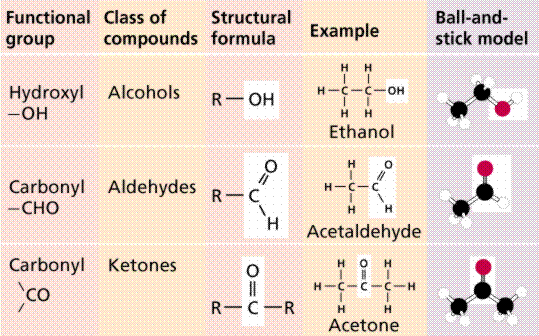
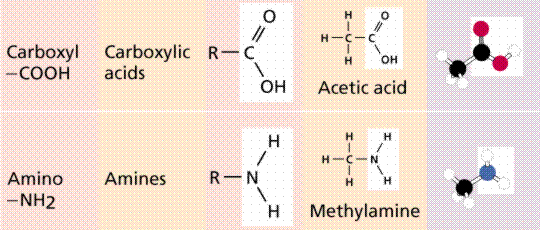
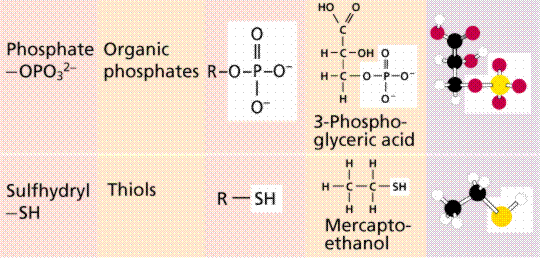
Functional groups in organic molecules.
Chemical bonds store energy. The C-C covalent bond has 83.1 Kcal (kilocalories) per mole, while the C=C double covalent bond has 147 Kcal/mole. Energy is in two forms: kinetic, or energy in use/motion; and potential, or energy at rest or in storage. Chemical bonds are potential energy, until they are converted (remember the laws of thermodynamics).
There are four major groups of organic macromolecules.
1. Carbohydrates have the general formula [CH2O]n. Note the different CH2O units on the diagram below. The main function of carbohydrates is for short-term energy storage (such as sugar). A secondary function is intermediate-term energy storage (as in starch for plants and glycogen for animals). Some carbohydrates are involved as structural components in cells, such as cellulose in the cell walls of plants and many protists.
Sugars are structurally the simplest carbohydrates. They are the structural unit which makes up the other classes of carbohydrates. Monosaccharides are single (mono) sugars. Important monosaccharides include ribose (C5H10O5), glucose (C6H12O6), and fructose (same formula but different structure than glucose).
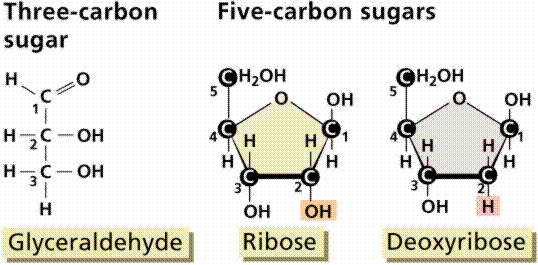
The chain (left) and ring (center and right) method of representing carbohydrates.
Classification of monosaccharides is done by the number of carbon atoms and the types of functional groups. For example, ribose and ribulose have the same formula, but different structure: ribose having an aldehyde (internal hydroxyl shown as: -OH) and ribulose having a keto group (internal double-bond O, shown as: =O).
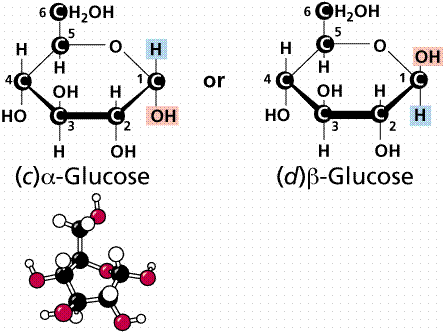
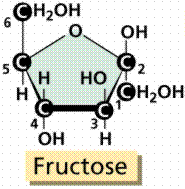
Models of glucose and fructose. .
In aqueous solution, glucose tends to have two structures, a and b, with an intermediate straight-chain form. The a form and b form differ in the location of one -OH group. Glucose is a common hexose in plants. The products of photosynthesis are assembled to make a glucose. Energy from sunlight is converted into the C-C covalent bond energy. This energy is released in living organisms in such a way that not enough heat is generated at once to incinerate the organisms. One mole of glucose yields 673 Kcal of energy. (A calorie is the amount of heat needed to raise one gram of water 1 degree C. A Kcal has 1000 times as much energy as a cal.)
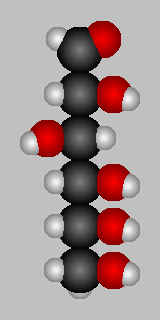
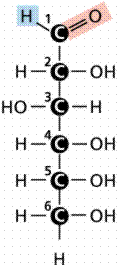
D-Glucose in various views (stick and space-filling) from the web.
Disaccharides are formed when two monosaccharides are chemically bonded together. Sucrose, a common plant disaccharide is composed of the monosaccharides glucose and fructose. Lactose, milk sugar, is a disaccharide composed of glucose and the monosaccharide galactose.
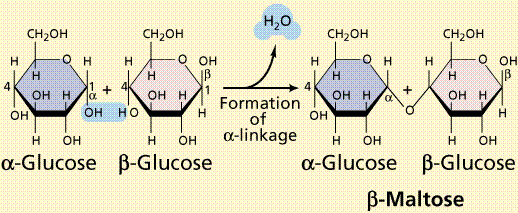
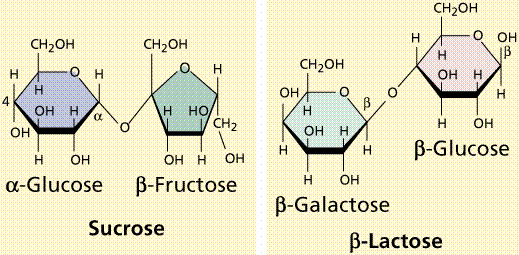
Formation of a disaccharide (top) by condensation and structure of two common disaccharides.
Polysaccharides are large molecules composed of individual monosaccharide units. A common plant polysaccharide is starch, which is made up of many glucoses (in a polypeptide these are referred to as glucans). Two forms of polysaccharide, amylose and amylopectin makeup what we commonly call starch. The formation of the ester bond by condensation (the removal of water from a molecule) allows the linking of monosaccharides into disaccharides and polysaccharides. Glycogen is an animal storage product that accumulates in the vertebrate liver.
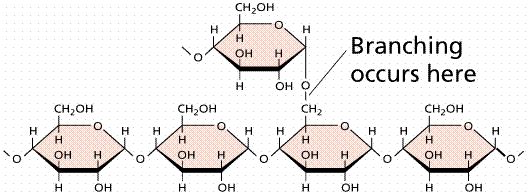
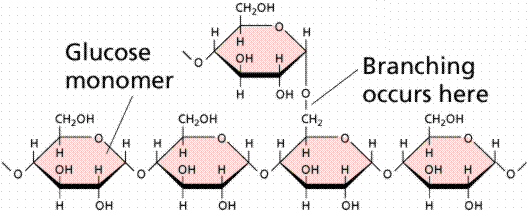
Images of starch (top), glycogen (middle), and cellulose (bottom).
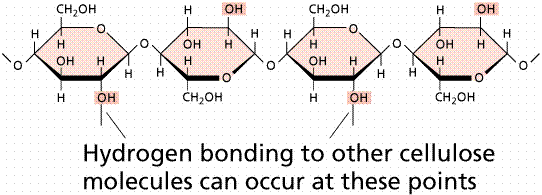
Cellulose is a polysaccharide found in plant cell walls. Cellulose forms the fibrous part of the plant cell wall. In terms of human diets, cellulose is indigestible, and thus forms an important, easily obtained part of dietary fiber. As compared to starch and glycogen, which are each made up of mixtures of a and b glucoses, cellulose (and the animal structural polysaccharide chitin) are made up of only b glucoses. The three-dimensional structure of the structural polysaccharides is thus constrained into straight microfibrils by the uniform nature of the glucoses, which resist the actions of enzymes (such as amylase) that breakdown storage polysaccharides (such a starch).
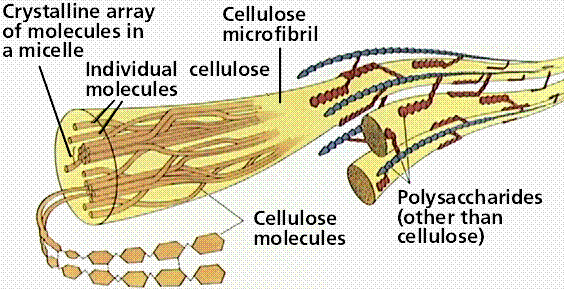
Structure of cellulose as it occurs in a plant cell wall.
2. Lipids are involved mainly with long-term energy storage. They are generally insoluble in polar substances such as water. Secondary functions of lipids are as structural components (as in the case of phospholipids that are the major building block in cell membranes) and as "messengers" (hormones) that play roles in communications within and between cells. Lipids are composed of three fatty acids (usually) covalently bonded to a 3-carbon glycerol. The fatty acids are composed of CH2 units, and are hydrophobic/not water soluble.
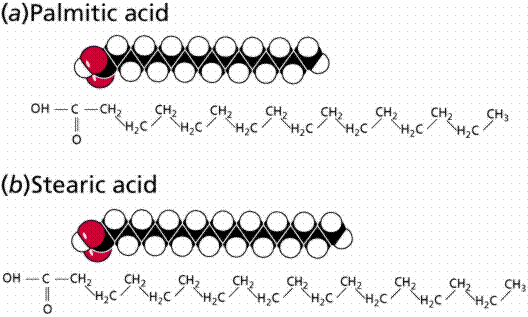
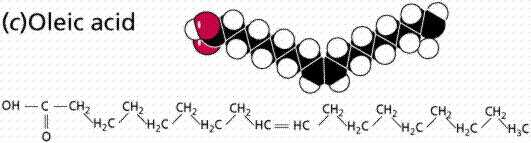
Saturated (top and middle) and unsaturated (bottom) fatty acids. The term staurated refers to the "saturation" of the molecule by hydrogen atoms. The presence of a double C=C covalent bond reduces the number of hydrogens that can bond to the carbon chain, hence the application of therm "unsaturated".
Fatty acids can be saturated (meaning they have as many hydrogens bonded to their carbons as possible) or unsaturated (with one or more double bonds connecting their carbons, hence fewer hydrogens). A fat is solid at room temperature, while an oil is a liquid under the same conditions. The fatty acids in oils are mostly unsaturated, while those in fats are mostly saturated.
Fats and oils function for in energy storage. Animals convert excess sugars (beyond their glycogen storage capacities) into fats. Most plants store excess sugars as starch, although some seeds and fruits have energy stored as oils (e.g. corn oil, peanut oil, palm oil, canola oil, and sunflower oil). Fats yield 9.3 Kcal/gm, while carbohydrates yield 3.79 Kcal/gm. Fats store six times as much energy as glycogen.
Diets are attempts to reduce the amount of fats present in specialized cells known as adipose cells that accumulate in certain areas of the human body. By restricting the intakes of carbohydrates and fats, the body is forced to draw on its own stores to makeup the energy debt. The body responds to this by lowering its metabolic rate, often resulting in a drop of "energy level." Successful diets usually involve three things: decreasing the amounts of carbohydrates and fats; exercise; and behavior modification.
Another use of fats is as insulators and cushions. The human body naturally accumulates some fats in the "posterior" area. Subdermal ("under the skin") fat plays a role in insulation.
Phospholipids and glycolipids are important structural components of cell membranes. Phospholipids are modified so that a phosphate group (PO4-) is added to one of the fatty acids. The addition of this group makes a polar "head" and two nonpolar "tails". Waxes are an important structural component for many organisms, such as the cuticle, a waxy layer covering the leaves and stems of many land plants; and protective coverings on skin and fur of animals.
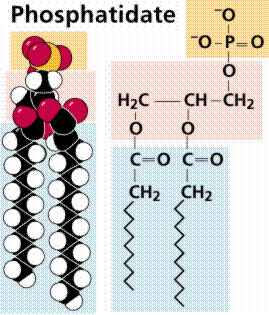
Structure of a phospholipid, space-filling model (left) and chain model (right).
Cholesterol and steroids: Most mention of these two in the news is usually negative. Cholesterol has many biological uses, such as its occurrence in the cell membranes, and its role in forming the sheath of some neurons. Excess cholesterol in the blood has been linked to atherosclerosis, hardening of the arteries. Recent studies suggest a link between arterial plaque deposits of cholesterol, antibodies to the pneumonia-causing form of Chlamydia, and heart attacks. The plaque increases blood pressure, much the way blockages in plumbing cause burst pipes in old houses.
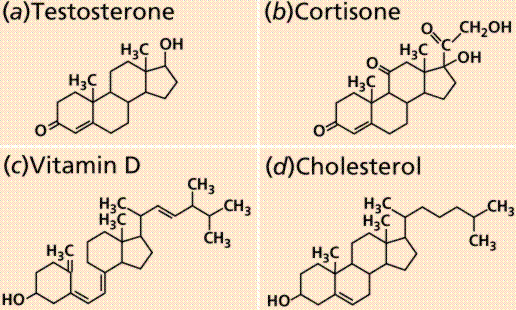
Structure of four steroids.
3. Proteins are very important in biological systems as control and structural elements. Control functions of proteins are carried out by enzymes and proteinaceous hormones. Enzymes are chemicals that act as organic catalysts (a catalyst is a chemical that promotes but is not changed by a chemical reaction). Click here for an illustrated page about enzymes. Structural proteins function in the cell membrane, muscle tissue, etc. The building block of any protein is the amino acid, which has an amino end (NH2) and a carboxyl end (COOH). The R indicates the variable component (R-group) of each amino acid. Alanine and Valine, for example, are both nonpolar amino acids, but they differ, as do all amino acids, by the composition of their R-groups. All living things (and even viruses) use various combinations of the same twenty amino acids. A very powerful bit of evidence for the phylogenetic connection of all living things.
Amino acids are linked together by joining the amino end of one molecule to the carboxyl end of another. Removal of water allows formation of a type of covalent bond known as a peptide bond.
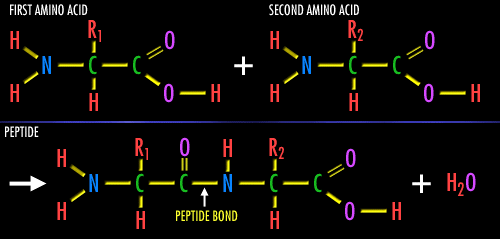
Formation of a peptide bond between two amino acids by the condensation (dehydration) of the amino end of one amino acid and the acid end of the other amino acid. The above image is from http://zebu.uoregon.edu/internet/images/peptide.gif.
Amino acids are linked together into a polypeptide, the primary structure in the organization of proteins. The primary structure of a protein is the sequence of amino acids, which is directly related to the sequence of information in the RNA molecule, which in turn is a copy of the information in the DNA molecule. Changes in the primary structure can alter the proper functioning of the protein. Protein function is usually tied to their three-dimensional structure. The primary structure is the sequence of amino acids in a polypeptide.
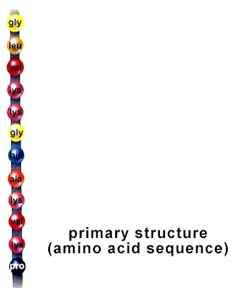
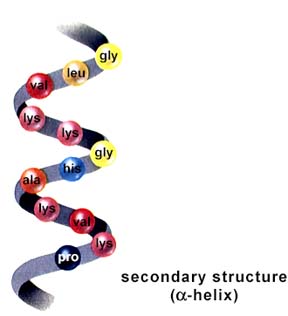
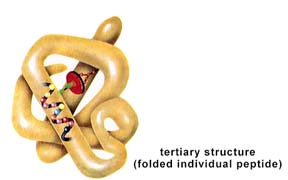
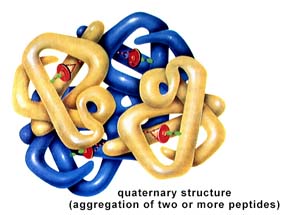
Structure of a protein: primary, secondary, tertiary, and quaternary levels of structure. The above images are from http://www.biosci.uga.edu/almanac/bio_103/notes/may_14.html.
The secondary structure is the tendency of the polypeptide to coil or pleat due to H-bonding between R-groups. The tertiary structure is controlled by bonding (or in some cases repulsion) between R-groups. Many proteins, such as hemoglobin, are formed from one or more polypeptides. Such structure is termed quaternary structure. Structural proteins, such as collagen, have regular repeated primary structures. Like the structural carbohydrates, the components determine the final shape and ultimately function. Collagens have a variety of functions in living things, such as the tendons, hide, and corneas of a cow. Keratin is another structural protein. It is found in fingernails, feathers, hair, and rhinoceros horns. Microtubules, important in cell division and structures of flagella and cilia (among other things), are composed of globular structural proteins.
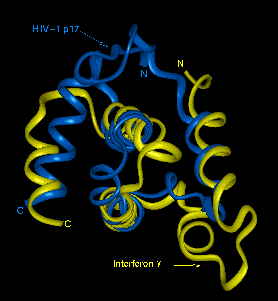
The above image is from http://nmra.ocms.ox.ac.uk/public/pdb/p17/p17.html and shows HIV p17 protein and similarities of its structure to gamma interferon.
4. Nucleic acids are polymers composed of monomer units known as nucleotides. There are a very few different types of nucleotides. The main functions of nucleotides are information storage (DNA), protein synthesis (RNA), and energy transfers (ATP and NAD). Nucleotides consist of a sugar, a nitrogenous base, and a phosphate. The sugars are either ribose or deoxyribose. They differ by the lack of one oxygen in deoxyribose. Both are pentoses usually in a ring form. There are five nitrogenous bases. Purines (Adenine and Guanine) are double-ring structures, while pyrimidines (Cytosine, Thymine and Uracil) are single-ringed.
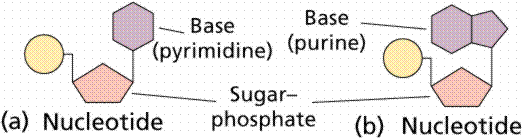
Structure of two types of nucleotide.
Deoxyribonucleic acid (better known as DNA) is the physical carrier of inheritance for 99% of living organisms. The bases in DNA are C, G, A and T. We will learn more about the DNA structure and function later in the course (click here for a quick look [actually take all the time you want!] ;)). DNA functions in information storage. The English alphabet has 26 letters and over 50,000 words. DNA has 4 letters (C, G, A, and T) and 20 words (the 20 amino acids) that can make an infinite variety of sentences (polypeptides). Changes in information can alter the meaning of a sentence.
For example take the sentence: I saw Elvis. This implies certain knowledge (that I've been out in the sun too long without a hat, etc.).
If we alter the sentence by inverting the middle word, we get: I was Elvis (thank you, thank you very much). Now we have greatly altered the information.
A third alteration will change the meaning: I was Levis. Clearly the original sentence's meaning is now greatly changed.
Changes in DNA information will be translated into changes in the primary structure of a polypeptide, and from there to the secondary and tertiary structures. A mutation is any change in the DNA base sequence. Most mutations are harmful, few are neutral, and a very few are beneficial and contribute the organism's reproductive success. Mutations are the wellspring of variation, variation is central to Darwin and Wallace's theory of evolution by natural selection.
Ribonucleic acid (RNA) was discovered after DNA. DNA, with exceptions in chloroplasts and mitochondria, is restricted to the nucleus (in eukaryotes, the nucleoid region in prokaryotes). RNA occurs in the nucleus as well as in the cytoplasm (also remember that it occurs as part of the ribosomes that line the rough endoplasmic reticulum). There are three types of RNA:
Messenger RNA (mRNA) is the blueprint for construction of a protein.
Ribosomal RNA (rRNA) is the construction site where the protein is made.
Transfer RNA (tRNA) is the truck delivering the proper amino acid to the site at the right time.
Details of RNA and its role in protein synthesis are available by clicking here.
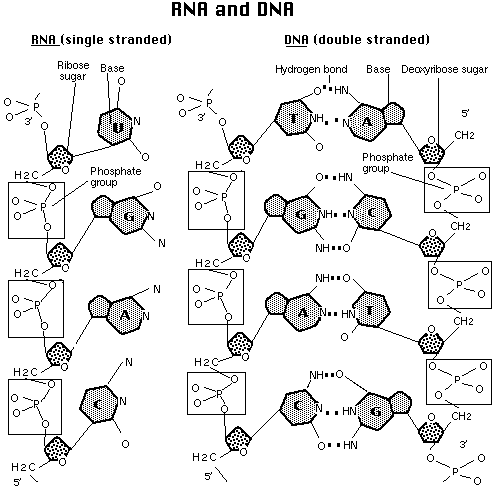
The diagram above is from the Access Excellence site (http://outcast.gene.com/ae//AB/GG/rna.html)
These learning objectives are taken from my Biology for Nonmajors class (BIO 102). I have tried to add a link to each that will direct you to a part of this chapter or another website that will facilitate your completion of the objective.
Back to Table of Contents | Go To CELLS I: ORIGINS
Email: mj.farabee@emcmail.maricopa.edu![]()
Last modified:
2000/01/05:08:54:11
The URL of this page is:
gened.emc.maricopa.edu/bio/BIO181/BIOBK/BioBookCHEM2.html