Evolution of Plants | The Plant Life Cycle | Plant Adaptations to Life on Land
Bryophytes | Tracheophytes: The Vascular Plants | Vascular Plant Groups | The Psilophytes | The Lycophytes
The Equisetophytes | The Ferns | Links
The plant kingdom contains multicellular phototrophs that usually live on land. The earliest plant fossils are from terrestrial deposits, although some plants have since returned to the water. All plant cells have a cell wall containing the carbohydrate cellulose, and often have plastids in their cytoplasm. The plant life cycle has an alternation between haploid (gametophyte) and diploid (sporophyte) generations. There are more than 300,000 living species of plants known, as well as an extensive fossil record.
Plants divide into two groups: plants lacking lignin-impregnated conducting cells (the nonvascular plants) and those containing lignin-impregnated conducting cells (the vascular plants). Living groups of nonvascular plants include the bryophytes: liverworts, hornworts, and mosses. Vascular plants are the more common plants like pines, ferns, corn, and oaks.
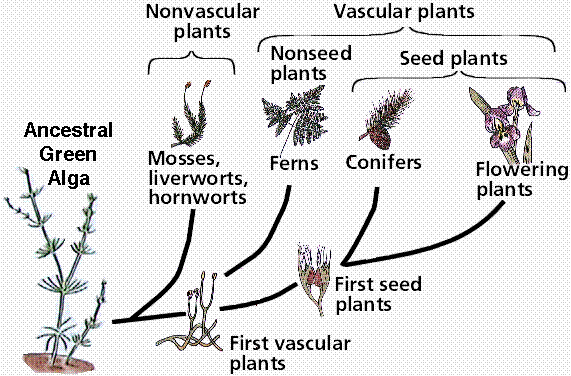
Phylogenetic reconstruction of the possible relationships between plant groups and their green algal ancestor. Note this drawing proposes a green algal group, the Charophytes, as possible ancestors for the plants.
Fossil and biochemical evidence indicates plants are descended from multicellular green algae. Various green algal groups have been proposed for this ancestral type, with the Charophytes often being prominently mentioned. Cladistic studies support the inclusion of the Charophytes (including the Coleochaetales) as sister taxa to the land plants. Algae dominated the oceans of the precambrian time over 700 million years ago. Between 500 and 400 million years ago, some algae made the transition to land, becoming plants by developing a series of adaptations to help them survive out of the water.
Table 1. Photosynthetic pigments of algae and plants. Prokaryote groups are shown in red, protists in blue, and vascular plants in purple.
|
|
|
|
Cyanobacteria |
chlorophyll a, chlorphyll c, phycocyanin, phycoerythrin |
|
Chloroxybacteria |
chlorophyll a, chlorphyll b |
|
Green Algae (Chlorophyta) |
chlorophyll a, chlorphyll b, carotenoids |
|
Red Algae (Rhodophyta) |
chlorophyll a, phycocyanin, phycoerythrin, phycobilins |
|
Brown Algae (Phaeophyta) |
chlorophyll a, chlorphyll c, fucoxanthin and other carotenoids |
|
Golden-brown Algae (Chrysophyta) |
chlorophyll a, chlorphyll c, fucoxanthin and other carotenoids |
|
Dinoflagellates (Pyrrhophyta) |
chlorophyll a, chlorphyll c, peridinin and other carotenoids |
|
Vascular Plants |
chlorophyll a, chlorphyll b, carotenoids |
Stemmed, or vascular, plants appeared by 350 million years ago, with forests soon following (300 million years ago). Seed plants next evolved, with flowering plants appearing around 140 million years ago.
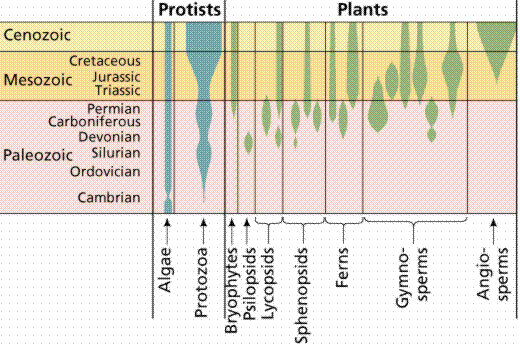
The fossil records of some protist and plant groups. The width of the shaded space is an indicator of the number of species.
Plants have an alternation of generations: the diploid spore-producing plant (sporophyte) alternates with the haploid gamete-producing plant (gametophyte). Animal life cycles have meiosis followed immediately by gametogenesis. Gametes are produced directly by meiosis. Male gametes are sperm. Female gametes are eggs or ova.
The plant life cycle has mitosis occurring in spores, produced by meiosis, that germinate into the gametophyte phase. Gametophyte size ranges from three cells (in pollen) to several million (in a "lower plant" such as moss). Alternation of generations occurs in plants, where the sporophyte phase is succeeded by the gametophyte phase. The sporophyte phase produces spores by meiosis within a sporangium. The gametophyte phase produces gametes by mitosis within an antheridium (producing sperm) and/or archegonium (producing eggs). Within the plant kingdom the dominance of phases varies. Nonvascular plants, the mosses and liverworts, have the gametophyte phase dominant. Vascular plants show a progression of increasing sporophyte dominance from the ferns and "fern allies" to angiosperms.
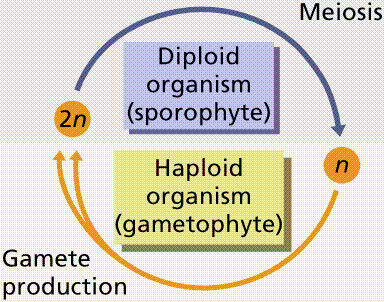
Typical alternation of generations life cycle, such as occur in some protistans and plants.
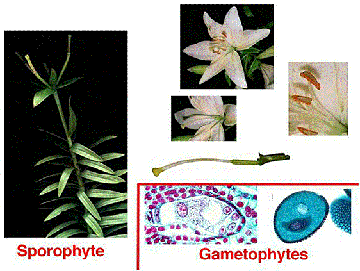
The above image is reduced from gopher://wiscinfo.wisc.edu:2070/I9/.image/.bot/.130/Angiosperm/Angiosperm_life_cycle. Follow that link to view a larger image.
Homospory and Heterospory
Plants have two further variations on their life cycles. Plants that produce bisexual gametophytes have those gametophytes germinate from isospores (iso=same) that are about all the same size.
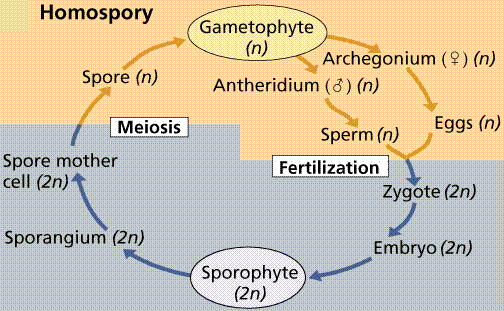
A typical homosporous life cycle. Note the production of a siungle type of bisexual gametophyte that will eventually produce the antheridia (sperm bearing structures) and archegonia (egg bearing structures).
Plants that produce separate male and female gametophytes have those gametophytes germinate from (or within in the case of the more advanced plants) spores of different sizes (heterospores; hetero=different). The male gametophyte produces sperm, and is associated with smaller or microspores. The female gametophyte is associated with the larger or megaspores. Heterospory is considered by botanists as a significant step toward the development of the seed.
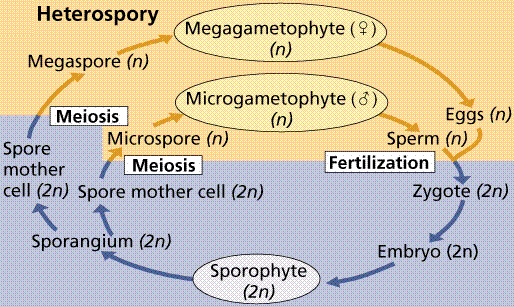
Typical heterosporous life cycle. Note the production of separate, unisexual male and female gametophytes.
Organisms in water do not face many of the challenges that terrestrial creatures do. Water supports the organism, the moist surface of the creature is a superb surface for gas exchange, etc. For organisms to exist on land, a variety of challenges must be met.
Bryophytes are small, nonvascular plants that first evolved approximately 500 million years ago. The earliest land plants were most likely bryophytes. Bryophytes lack vascular tissue and have life cycles dominated by the gametophyte phase. The lack of conducting cells limits the size of the plants, generally keeping them under 5 inches high. Roots are absent in bryophytes, instead there are root-like structures known as rhizoids. The group includes the hornworts, liverworts, and mosses.
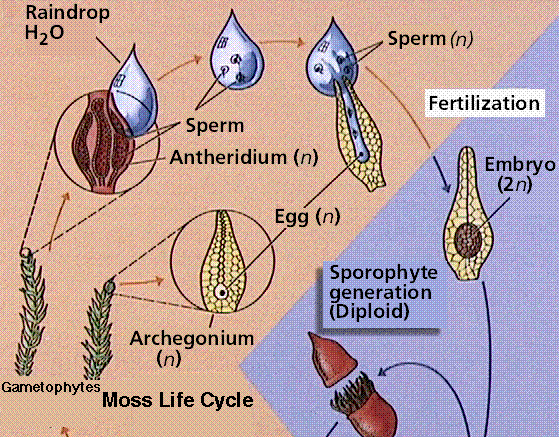
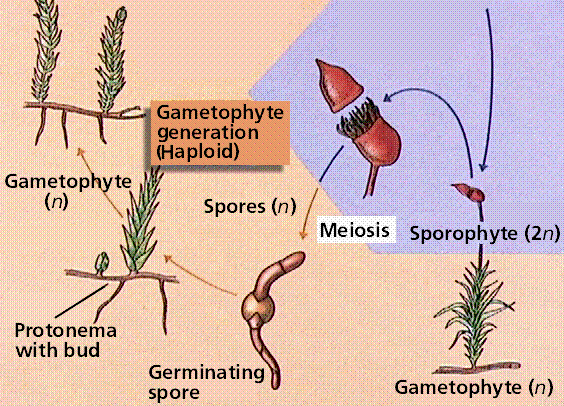
The moss life cycle. The haploid gametophyte phase is free-living and photosynthetic. The diploid sporophyte grows from and is nourished by the gametophyte.
The vascular plants have specialized transporting cells xylem (for transporting water and mineral nutrients) and phloem (for transporting sugars from leaves to the rest of the plant). When we think of plants we invariably picture vascular plants. Vascular plants tend to be larger and more complex than bryophytes, and have a life cycle where the sporophyte is more prominent than the gametophyte. Vascular plants also demonstrate increased levels of organization by having organs and organ systems.
Table 2. Major evolutionary advances of the vascular plants.
|
Advance |
Green Algae |
Bryophytes |
Tracheophytes |
|
Development of the root-stem-leaf vascular system |
nonvascularized body (thallus) that may be variously shaped no levaes, shoots, or roots |
no vascular system leaflike structures are present, but lack any vascular tissue |
early vascular plants are naked, rootless vascularized stems later vascular plants develop vascularized leaves, then roots |
|
Reduction in the size of the gametophyte generation |
wide range of life cycles, some gametophyte dominant, others sporophyte dominant |
sporophyte generation dependant on gametophyte generation for food; gametophyte is free-living and photosynthetic |
progressive reduction in size and complexity of the gametoiphyte generation, leading to its complete dependance on the sporophyte for food in angiosperms, 3 celled male gametophyte and a (usually) 8 celled female gametophyte |
|
Development of seeds in some vascular plants |
no seeds |
no seeds |
seed plants retain the female gametophyte on the sporophyte |
|
Spores/Pollen |
spores for resisting environmental degradation |
Spores that germinate into the gametophyte generation |
Spores that germinate into the gametophyte generation or spores that have the gametophyte generation develop within themselves |
Vascular plants developed during the Silurian Period, 400 million years ago. The earliest vascular plants had no roots, leaves, fruits, or flowers.
Cooksonia is a typical early vascular plant. It was less than 15 cm tall, with stems that dichotomously branched. Dichotomous branching (where the stem divides into two ewqual branches) appears a primitive or ancestral trait in vascular plants. Some branches terminated in sporangia that produced a single size of spore.
Many scientists now consider "Cooksonia" an evolutionary grade rather than a true monophyletic taxon. Their main argument is that not all stems of Cooksonia-type plants have vascular tissue. The evolutionary situation of a grade would have some members of the group having the trait, others not. The shapes of sporangia on various specimens of Cooksonia also vary considerably.

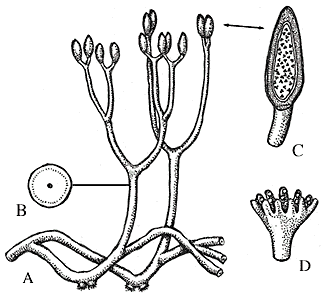
Cooksonia fossil specimen (L) and reconstruction (R). Both Images from http://www.ucmp.berkeley.edu.
Rhynia is another early vascular plant. Like Cooksonia, it lacked leaves and roots. One of the species formerly assigned to this genus, R. major, has since been reclassified as Aglaophyton major. Some paleobotanists consider A. major a bryophyte, however, it does have a separate free-living sporophyte that is more prominent than the sporophyte, but appears to lack lignified conducting cells. The remaining species, R. gwynne-vaughanii is an undoubted vascular plant.
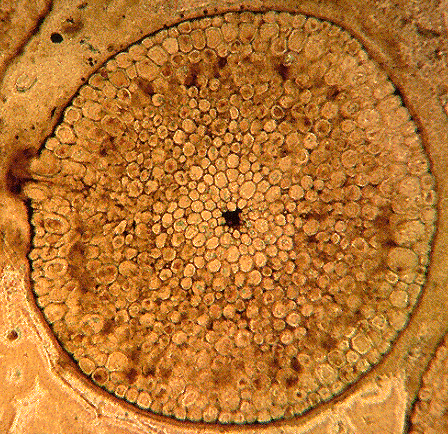
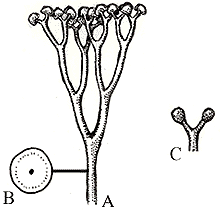
Rhynia gwynne-vaughanii (L) stem x.s. of, from the Rhynie Chert in Scotland, image cropped and reduced from http://www.uni-muenster.de/GeoPalaeontologie/Palaeo/Palbot/rhynie.html. (R) Reconstruction of the plant, from http://www.ucmp.berkeley.edu/IB181/VPL/Elp/Elp2.html.
Reconstruction of Aglaophyton major (A-C) and Lyonophyton rhyniensis, another Rghynie Chert plant thought to be the gametophyte of Aglaophyton.
Devonian plant lines included the trimerophytes and zosterophyllophytes.
Psilotum nudum (the whisk fern) is a living plant that somewhat resembles what paleobotanists believe Cooksonia to have been: a naked, photosynthetic stem with sporangia. However, most paleobotanists doubt that Psilotum is a descendant of Cooksonia. Psilotum has three fused sporangia, termed a synangium, located on the sides instead of the tips of stems (as in Cooksonia).

Psilotum nudum from Hawaii. Image from http://www.botany.hawaii.edu/faculty/carr/images/psi_nud_mid.jpg.
The next group, the Lycophyta, have their sporangia organized into strobili (sing.: strobilus). Leaves that contained vascular tissue are another major advance for this group.
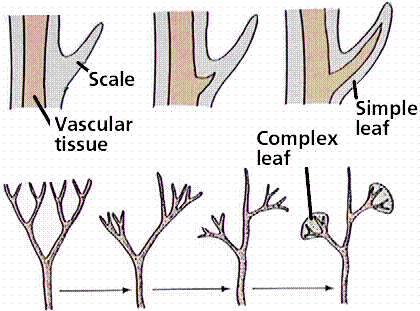
Proposed steps in the evolution of the microphyll leaf. Note that microphylls do not leave a leaf gap in the stem's vascular cylinder. If we wanted to place Psilotum-like plants on the left, we would have Lycopodium-like plants on the right.
Today there are much fewer genera of lycophytes than in the Paleozoic Era. Major living lycophytes include Lycopodium (commonly called the club moss, although it is NOT a moss), Isoetes, and Selaginella (the resurrection plant). Lycopodium produces isospores that germinate in the soil and produce a bisexual gametophyte. These spores are all about the same size. Selaginella and Isoetes produce two sizes of spores: small spores (microspores) that germinate to produce the male gametophyte; and larger spores (megaspores) that germinate to produce the female gametophyte. The production of two sizes of spores, and also making separate unisexual gametophytes, is thought an important step toward the seed. Modern lycophytes are small, herbaceous plants. Many of the prominent fossil members of this group produced wood and were significant trees in the Carboniferous-aged coal swamps.

Lycopodium venustum, from from Hawaii. Image from http://www.botany.hawaii.edu/faculty/carr/images/lyc_ven.jpg.
Fossil Lycophytes: Baragwanathia and Drepanophycus
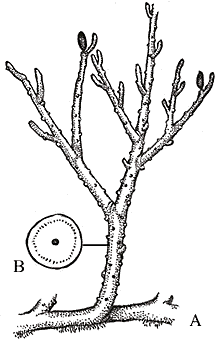
Baragwanathia is an undoubted lycophyte from the middle Silurian deposits of Australia. It has microphyllous leaves spirally attached to the stem, and sporangia clustered in some areas of the plant, although not in terminal strobili.
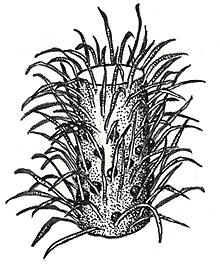
Reconstruction of Baragwanathia longifolia, from the middle Silurian of Australia. Image from http://www.ucmp.berkeley.edu/IB181/VPL/Lyco/Lyco1.html.
Drepanophycus is a middle Devonian lycophyte from the Northern Hemisphere. Its features are very similar to modern lycophytes.
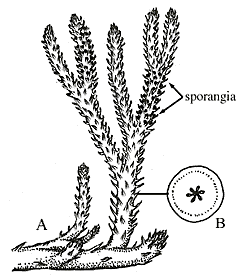
Reconstruction of Drepanophycus , a middle Devonian lycophyte. Note the numerous microphyll leaves, placement of sporangia on the upper surface of the sporophylls, and stem anatomy that are all consistent with modern lycophytes. Image from http://www.ucmp.berkeley.edu/IB181/VPL/Lyco/Lyco2.html.
Lepidodendron and Sigillaria
Once dominant elements in the Paleozoic forests, equisetophytes are today relegated to minor roles as herbaceous plants. The group is defined by their jointed stems, with many leaves being produced at a node, production of isospores in cones borne at the tips of stems, and spores bearinmg elaters (devices to aid in spore dispersal). The gametophyte is small, photosynthetic and free-living. Silica concentrated in the stems give this group one of their common names: scouring rushes since they were reportedly used by American pioneers to scour the pots and pans. The fossil members of this group are often encountered in coal deposits of Carbonifersous age in North America and Europe.

A branched species of Equisetum from Hawaii. Note that the branches arise from the same node/area along the stem. Image from http://www.botany.hawaii.edu/faculty/carr/images/equ_sp.jpg.

Closeup view of the cone of Equisetum hyemale from Hawaii. Note the small leaves at the joints near the lettering in the picture. Image from http://www.botany.hawaii.edu/faculty/carr/images/equ_sp_cu.jpg.
Ferns reproduce by spores from which the free-living bisexual gametophyte generation develops. There are 12,000 species of ferns today, although the fossil history of ferns shows them to have been a dominant plant group during the Paleozoic Era. Ferns have megaphyllous leaves, which cause a leaf gap in the vascular cylinder of the stem/rhizome.
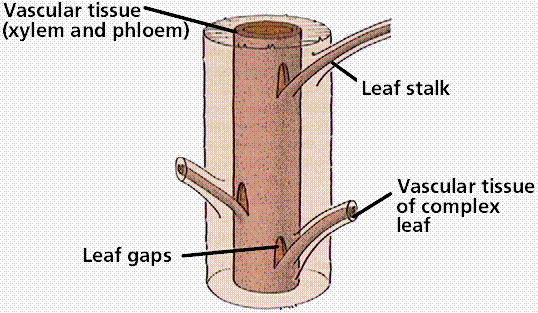
Formation of leaf gaps by a megaphyllous leaf. Most plants above the ferns have megaphyllous leaves.

Three genera of the fern family Gleicheniaceae from Hawaii: Upper left: Sticheris owhyhensis, lower left: Diploterygium pinnatum (uluhe lau nui), right: Dicranopteris linearis (uluhe). Image from http://www.botany.hawaii.edu/faculty/carr/gleicheni.htm.
The Fern Life Cycle
The fern gametophyte has both sexes present and is referred to as a prothallium. Prothallia develop from spores shed from the underside of the sporophyte leaves.Once fertilization occurs, the next generation sporophyte develops from the egg located in the prothallium.

Spoprangia on the underside of Polypodium pellucidum from Hawaii. Image from http://www.botany.hawaii.edu/faculty/carr/images/pol_pel.jpg.
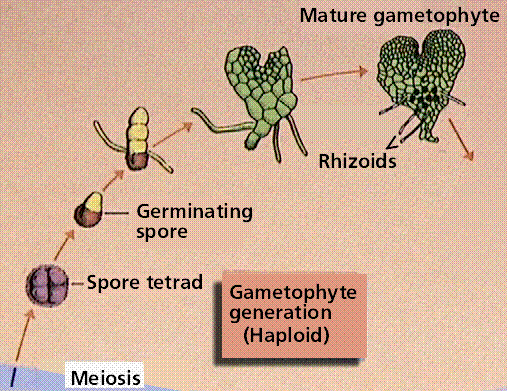
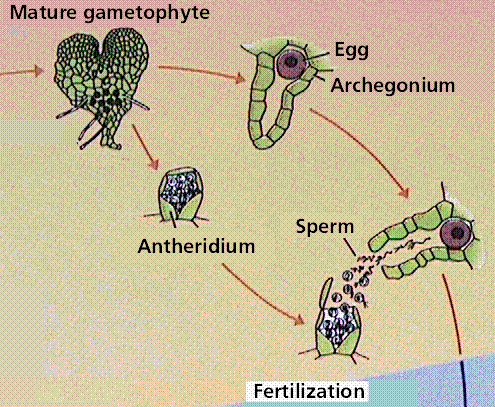
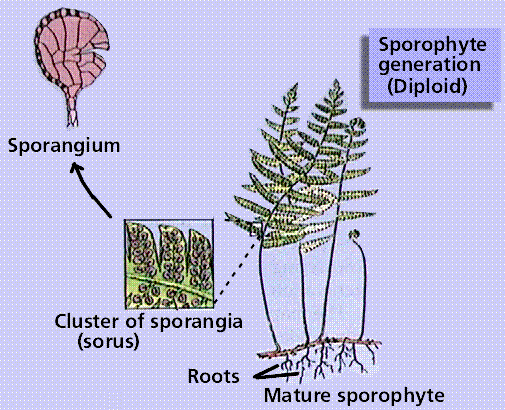
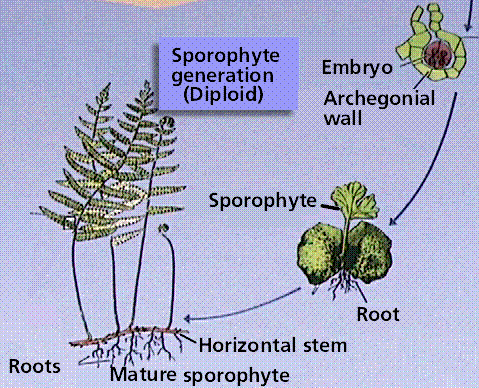
Composite of 4 segmented diagrams of the fern life cycle. Note: to view this in its proper sequence you will need to open your browsert window as wide as possible.
Email: mj.farabee@emcmail.maricopa.edu![]()
Last modified:
2000/01/05:09:27:19
The URL of this page is:
gened.emc.maricopa.edu/bio/BIO181/BIOBK/BioBookDiversity_5.html