Carrier-assisted Transport | Types of transport molecules | Vesicle-mediated transport
Cell membranes act as barriers to most, but not all, molecules. They are thus differentially (or semi-) permeable. Water potential is the tendency of water to move from an area of higher concentration to one of lower concentration. Energy exists in two forms: potential and kinetic. Water molecules move according to differences in potential energy between where they are and where they are going. Gravity and pressure are two enabling sources for this movement. Remember in the hydrologic cycle that water runs downhill (likewise it falls from the sky, to get into the sky it must be acted on by the sun and evaporated, thus needing energy input to power the cycle).
Bulk flow is the overall movement of a fluid. This moves liquid throughout a multicellular individual. Diffusion is the net movement of a substance (liquid or gas) from an area of higher concentration to one of lower concentration. You are on a large (10 ft x 10 ft x10 ft) elevator. An obnoxious individual with a lit cigar gets on at the third floor with the cigar still burning. You are also unfortunate enough to be in a very tall building and the person says "Hey we're both going to the 62nd floor!" Disliking smoke you move to the farthest corner you can. Eventually you are unable to escape the smoke! An example of diffusion in action. Nearer the source the concentration of a given substance increases.
Since the molecules of any substance (solid, liquid, or gas) are in motion when that substance is above absolute zero (0 degrees Kelvin or -273 degrees C), there is available energy for movement of the molecules from a higher potential state to a lower potential state, just as in the case of the water discussed above. The majority of the molecules move from higher to lower concentration, although there will be some that move from low to high. The overall (or net) movement is thus from high to low concentration. Eventually, if no energy is input into the system the molecules will reach a state of equilibrium where they will be distributed equally throughout the system.
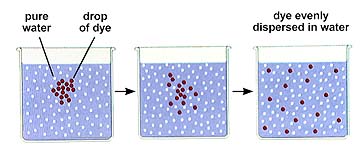
The above image is from http://www.biosci.uga.edu/almanac/bio_103/notes/may_13.html.
Water, carbon dioxide, and oxygen are among the few simple molecules that can cross the cell membrane by diffusion (or a type of diffusion known as osmosis ). Diffusion is one principle method of movement of substances within cells, as well as method for essential small molecules to cross the cell membrane. Gas exchange in gills and lungs operates by this process. Carbon dioxide is produced by all cells as a result of cellular metabolic processes to be detailed in a later unit. Since the source is inside the cell, the concentration gradient is constantly being replenished/re-elevated, thus the net flow of CO2 is out of the cell. Metabolic processes usually require oxygen, which is in greater concentration inside the cell, thus the net flow of oxygen is into the cell.
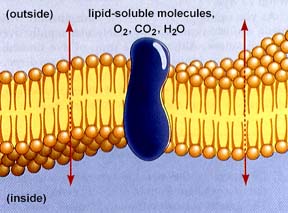
The above image is from http://www.biosci.uga.edu/almanac/bio_103/notes/may_13.html.
Osmosis is the diffusion of water across a semi-permeable (or differentially permeable or selectively permeable) membrane. The cell membrane, along with such things as dialysis tubing and cellulose acetate sausage casing, is such a membrane. The presence of a solute decreases the water potential of a substance. Thus there is more water per unit of volume in a glass of fresh-water than there is in an equivalent volume of sea-water. In a cell, which has so many organelles and other large molecules, the water flow is generally into the cell.
Hypertonic solutions are those in which more solute (and hence lower water potential) is present. Hypotonic solutions are those with less solute (again read as higher water potential). Isotonic solutions have equal (iso-) concentrations of substances. Water potentials are thus equal, although there will still be equal amounts of water movement in and out of the cell, the net flow is zero.
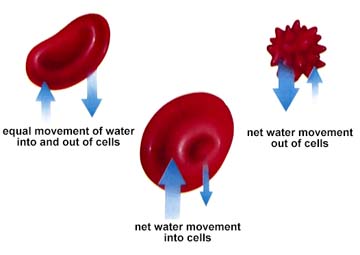
The above image is from http://www.biosci.uga.edu/almanac/bio_103/notes/may_13.html.
One of the major functions of blood in animals is the maintain an isotonic internal environment. This eliminates the problems associated with water loss or excess water gain in or out of cells. Again we return to homeostasis. Paramecium and other single-celled freshwater organisms have difficulty since they are usually hypertonic relative to their outside environment. Thus water will tend to flow across the cell membrane, swelling the cell and eventually bursting it. Not good for any cell! The contractile vacuole is the Paramecium's answer to this problem. The pumping of water out of the cell by this method requires energy since the water is moving against the concentration gradient.
Passive transport requires no energy from the cell. Examples include the diffusion of oxygen and carbon dioxide, osmosis of water, and facilitated diffusion.
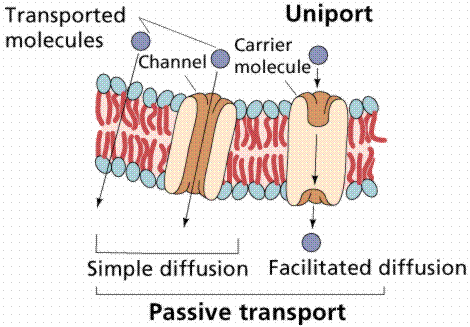
Image from W.H. Freeman and Sinauer Associates, used by permission.
Active transport requires the cell to spend energy, usually in the form of ATP. Examples include transport of large molecules (non-lipid soluble) and the sodium-potassium pump.

Image from W.H. Freeman and Sinauer Associates, used by permission.
The transport proteins integrated into the cell membrane are often highly selective about the chemicals they allow to cross. Some of these proteins can move materials across the membrane only when assisted by the concentration gradient, a type of carrier-assisted transport known as facilitated diffusion. Both diffusion and facilitated diffusion are driven by the potential energy differences of a concentration gradient. Glucose enters most cells by facilitated diffusion. There seem to be a limiting number of glucose-transporting proteins. The rapid breakdown of glucose in the cell (a process known as glycolysis) maintains the concentration gradient. When the external concentration of glucose increases, however, the glucose transport does not exceed a certain rate, suggesting the limitation on transport.
In the case of active transport, the proteins are having to move against the concentration gradient. For example the sodium-potassium pump in nerve cells. Na+ is maintained at low concentrations inside the cell and K+ is at higher concentrations. The reverse is the case on the outside of the cell. When a nerve message is propagated, the ions pass across the membrane, thus sending the message. After the message has passed, the ions must be actively transported back to their "starting positions" across the membrane. This is analogous to setting up 100 dominoes and then tipping over the first one. To reset them you must pick each one up, again at an energy cost. Up to one-third of the ATP used by a resting animal is used to reset the Na-K pump.
Uniport transports one solute at a time. Symport transports the solute and a cotransported solute at the same time in the same direction. Antiport transports the solute in (or out) and the co-transported solute the opposite direction. One goes in the other goes out or vice-versa.
Vesicles and vacuoles that fuse with the cell membrane may be utilized to release or transport chemicals out of the cell or to allow them to enter a cell. Exocytosis is the term applied when transport is out of the cell.

This GIF animation is from http://www.stanford.edu/group/Urchin/GIFS/exocyt.gif. Note the vesicle on the left, and how it fuses with the cell membrane on the right, expelling the vesicle's contents to the outside of the cell.
Endocytosis is the case when a molecule causes the cell membrane to bulge inward, forming a vesicle. Phagocytosis is the type of endocytosis where an entire cell is engulfed. Pinocytosis is when the external fluid is engulfed. Receptor-mediated endocytosis occurs when the material to be transported binds to certain specific molecules in the membrane. Examples include the transport of insulin and cholesterol into animal cells.
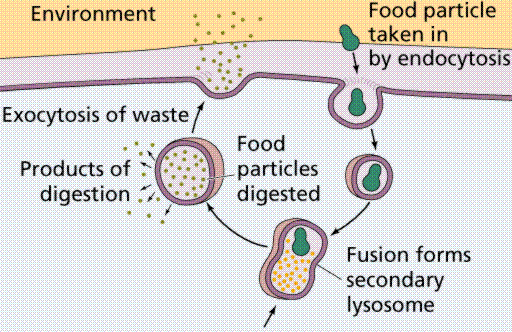
Image from W.H. Freeman and Sinauer Associates, used by permission.
Email: mj.farabee@emcmail.maricopa.edu![]()
Last modified: 2000/01/08:15:42:20
The URL of this page is: gened.emc.maricopa.edu/bio/BIO181/BIOBK/BioBooktransp.html