Ethics,
Biodiversity, and New Natural Products
Development
This
report was written by A.B.Cunningham as
part of the WWF/UNESCO/Kew «People and
Plants» Initiative. The report has been
endorsed by the International Society of
Ethnobiology. Any inaccuracies in the
report remain the responsibility of the
author. The opinions expressed are the
author’s and do not necessarily
reflect the views of WWF.
Author's
address:
A B
Cunningham
84 Watkins St, White Gum Valley,
Freemantle,
6162, AUSTRALIA
Edited
by Baxter Lindsay
Desktop
publishing by Madlen Tschopp
Produced
by the WWF International Publications
Unit
Published
April 1993 (under the title of Ethics,
Ethnobiological Research, and
Biodiversity), reprinted September 1996
by WWF-World Wide Fund For Nature
(formerly World Wildlife Fund), Gland,
Switzerland.
Any
reproduction in full or in part of this
publication must mention the title, and
credit the above-mentioned publisher as
the copyright owner.
Contents
Executive Summary
1.
Introduction
2. Objectives
3. Procedures and Key
Players in Chemical prospecting
4. The Issues Involved
4.1 Ethical and
Legal Approaches
4.2 Conservation and
Biodiversity
5. Wild Harvesting, Herbal
Preparations, and Extracts
6. Expectations and Economic
Benefits
7. Changes and Linkages
7.1 Researchers as
Expert Advisors
7.2 Researchers as
Brokers
7.3 Fitting Research
Objectives to National and
Local Priorities
8. Regional Returns
9. Policy and Principles for
Equitable Partnerships
10. Checks and Balances
11. Conclusion
Appendix
List of Acronyms
Glossary
References
Personal Communications
Acknowledgements
Ethics,
Biodiversity, and New Natural Products
Development: Guidelines for the Equitable
Use and Development of Indigenous
Knowledge and Biological Resources
Executive
Summary
In an ideal world,
biological diversity would be protected
for ethical, aesthetic, or spiritual
reasons. Forests, coral reefs, or
wetlands, for example, would be conserved
out of respect for other forms of life,
for their natural beauty, or for the
spiritual link provided between the human
species and the natural world.
Unfortunately, this is not the case.
Forests, coral reefs, and wetlands are
not being adequately conserved. They are
being replaced at an alarming rate by
monocultures of crops, pasture, algae, or
by construction projects.
Fortunately,
utilitarian values (monetary and
non-monetary) are playing an important
role in justifying the conservation of
biodiversity as a form of land use.
«Chemical prospecting» is a useful
argument in favour of conservation.
In an ideal academic
world, there would be a free flow of
knowledge for the good of all. But once
again, this is not the case. In the
development of natural products and in
«chemical prospecting», research
knowledge is often patented before it is
made public ensuring a flow of benefits
only to the patenting country or company.
Biodiversity will only
be relevant to national governments or
local people if a fair share of benefits
from new natural products is returned to
the region of origin. Until recently,
however, chemical resources - whether
derived from plants, corals or
micro-organisms - were considered global
common property. The fact is that
biodiversity is being destroyed because
it is undervalued, and chemical compounds
that are the precursors of new natural
products are no exception. To consider
chemical compounds of the wild habitat as
a global commons, freely available to
all, reduces their value to national
governments and to the local people who
are best placed to conserve them. For
this reason, clear guidelines are
required for the development of new
natural products.
National sovereignty is
recognized wherever oil, minerals,
timber, rattan, and several other
resources are found. Why should. it not
also apply to the chemical compounds that
come from species with limited
distribution? They are the starting
points for the development of new natural
products.
And what about the
indigenous knowledge that provides a key
to the active ingredients in plants or
fungi? Why shouldn't this be recognized
as the valuable product of an
intellectual and experimental process?
Why shouldn't local rights be attached to
this knowledge as well as the natural
compounds from biodiversity, before they
are made public?
These questions cannot
be resolved by ethnobiologists alone, but
only through a wider awareness of the
issues and actions required by
governments, chemists, industrial
companies, and the indigenous peoples
involved. For this reason, these
guidelines have been developed. They:
- outline the
ethical and conservation issues
that require the creation of
equitable partnerships in the
development of new natural
products; partnerships that
recognize and compensate for the
use of indigenous knowledge and
natural resources.
- facilitate
international cooperation in the
collection, conservation, use,
and development of new natural
products
- ensure that any
collecting for export and use
outside a country has the full
approval of the competent
authorities, and is carried out
with the cooperation of the host
country and representatives of
the local communities involved.
They also ensure that these
collections comply with
conservation and quarantine
regulations in the countries of
origin and destination
- outline the
general principles that will
facilitate the development of
national regulations by
governments or agreements between
organizations.
This is not a
futuristic issue. In 1991, the American
National Cancer Institute (NCI) awarded
three five-year collecting contracts
worth $3.8 million to two US botanical
gardens and the University of Illinois. A
similar five-year contract worth $2.9
million was awarded to the Coral Reef
Foundation for collecting marine
organisms for screening. Kew Gardens has
a contract from Glaxo to screen its
living plant collection for active
ingredients. In this way, scientific
activities are directly linked to
commercial interests. Understandably,
national governments in the tropical
countries concerned are asking "What
right do these organizations have to
'privatize' our resources?"
A code of practice is
suggested (see Appendix) that requires:
- Legislation to be
enacted at a regional or national
level to control the collection
and export of biological
material, with advice from
appropriate professional
organizations.
- A strict code of
professional ethics to ensure
that:
- research
participants (e.g.
traditional specialists) and
members of relevant local
organizations (e.g. herbaria)
are fully informed of the
objectives, commercial
aspects, and possible results
of research
- confidential
information and research
participants’, requests
for anonymity are respected.
- equitable
compensation is made for
assistance by individuals
- the relevant
national or regional
organization receives fair
royalty payments
- national
requirements for plant
collecting, including
collection with local
counterparts, are observed.
- Maximum use of
local expertise within developing
countries, or at regional level,
to undertake extraction and
screening of important compounds.
This should apply equally to
compounds of regional
significance (e.g. anti-fungus,
or anti-parasite) and global
significance (e.g.
anti-inflammation, anti-virus,
anti-cancer). This will involve a
commitment to training,
technology transfer, and the
development of practical, initial
screening techniques. It will
also require government support
for local scientists and research
organizations.
- Supply agreements
should only be made with
reputable organizations, not with
individuals whose primary
interest may be personal gain.
1.
Introduction
This document is a
research paper concerned with the ethical
issues that arise when new commercial
products are developed from biological
materials. The main part of the paper is
a discussion of the issues, and a
recommended code of practice is given in
the Appendix. Although the discussion
concentrates on plants, similar
principles apply to other organisms.
Comments on this paper should be sent to
the Biodiversity Unit, WWF International.
The bulk of the
world’s biological and cultural
diversity occurs in developing countries.
These are rich sources of potential new
natural products, and contain much
indigenous knowledge about plant and
animal uses. Although some developing
countries, such as India and Brazil, have
expanding pharmaceutical industries, much
of the technology and expertise required
to develop new industrial products from
biological materials is centered in the
industrialized countries of the temperate
zone. For many researchers who are
involved in recording indigenous
knowledge and identifying potentially
valuable biological resources, this
raises ethical, legal, and political
issues.
The need for socially
and environmentally responsible action
also applies to industrial companies,
corporations and government agencies.
Technological developments in genetic
engineering and biotechnology, and new
screening procedures for active
ingredients have all stimulated interest
amongst large companies which see new
industrial products arising from plants,
micro-organisms and marine organisms
(Weislow et al., 1989; Hamburger et
al., 1991).69,33 At the
same time, there is the realization that
both indigenous knowledge and biological
diversity are disappearing with cultural
and environmental change.
Three main issues have
been the focus of parallel debates
relating to professional ethics,
traditional knowledge and plant
conservation. First, international debate
since the early 1980s has focused on
local knowledge, farmers' rights, and
equity in the distribution and control of
genetic resources from crop plants
(Mooney, 1983; Kloppenberg, 1988).50,42
This issue has drawn heated
opposition from some prominent botanists
(Arnold et al., 1986),6
but was partially resolved when the
Commission on Plant Genetic Resources
(PGR) revised the FAO Undertaking for PGR
to recognize both plant-breeders’
and farmers’ rights (WRI, 1992).70
Second and more
recently, there has been debate relating
to the development of new natural
products from plants. There has been
focus on new pharmaceuticals, but the
issues are similar in the case of
development of new waxes, oils, perfumes,
and other products. (The issues are also
similar in the case of other types of
organisms, such as soil fungi, marine
organisms, and terrestrial animals.)
The third area of
debate has been the distribution of
economic benefits from plant species
with horticultural potential, such as
the African violet (Saintpaulia)
(Lovett, 1988).45
These debates hinge on:
- the recognition of
the intellectual contribution
made by farmers or specialist
plant users, such as herbalists,
beekeepers, and master fishermen,
to the development or
identification of crop land races
and new natural products
- the equitable
distribution of benefits from the
use of these crops, ornamentals,
wild plants, or their genetic or
chemical structures, to assist
people and the conservation of
biodiversity in their regions of
origin
- commitment to
technology transfer,
infrastructure development,
training programmes, and local
government support to enable the
development of crop varieties,
new natural products and
horticultural exports in the
regions of origin.
Debate on these issues
has also pointed to the linked interests
of multinational companies such as
Ciba-Geigy, Pfizer, and Monsanto in the
seeds, fertilizer and pharmaceuticals
industries (see Table 1 and Mooney,
1983).35,50 It has also called
into question the historic international
view that indigenous knowledge, as well
as chemical and genetic resources, is a
freely available global commons i.e. the
property of everyone. An unfortunate
result of regarding indigenous knowledge,
chemical structures, or genes as common
property is that these resources are seen
as belonging to nobody, and there is
little incentive to conserve either
species or habitats. This view has
recently changed.
Table 1. The world's
top ten pharmaceutical, seed and
pesticide companies showing dominance of
major companies in these fields and their
global sales values.
(USA = United States of
America; UK = United Kingdom: F = France:
G = Germany: NL = Netherlands: CH =
Switzerland)
Pharmaceuticals
|
Sales US$ billions
|
Seed Corporations
|
Sales US$
billions
|
Pesticides
|
Sales US$ billions
|
| Merck (USA) Smith
Kline-Beecham USA/UK)
Bristol
Myers-Squibb (USA)
Hoechst
(G)
Glaxo
(UK)
Rhone-Poulenc
Rorer (F/USA)
Ciba-Geigy
(CH)
Bayer
(G)
Am.
Home Products (USA)
Sandoz
(CH)
|
4.23
4.00
3.90
3.51
3.37
3.30
3.17
2.96
2.93
2.75
|
Pioneer
Hi-Bred (USA) Sandoz (CH)
Limagrain
(F)
Upjohn
(USA)
Aritrois
(F)
ICI
(UK)
Cargill
(USA)
Shell
(NL/UK)
Dekalb-Pfizer
(USA)
Ciba-Geigv
(CH)
|
0.73
0.51
0.37
0.28
0.26
0.25
0.23
0.20
0.17
0.15
|
Ciba-Geigy
(CH) Bayer (G)
ICI
(UK)
Rhone-Poulenc
(F)
Du
Pont (USA)
Dow
Elcano* (USA)
Monsanto
(USA)
Hoechst
(G)
BASF
(G)
Shell
(NL/UK)
|
2.14
2.07
1.96
1.63
1.44
1.42
1.38
1.02
1.00
0.94
|
| Total Sales
(top 10) |
34.12
|
|
3.10
|
|
5.00
|
| Global
Sales |
120.00
|
|
15.00
|
|
20.00
|
From Hobbelinh 199135
* Elanco was formed by
the merger between Eli Lilley and Dow
Chemicals
In 1989, UNESCO (see
List of Acronyms) adopted a resultion on
the Safeguarding of Traditional Culture
and Folklore (UNESCO, 1989).66
In 1991, the FAO Commission on Plant
Genetic Resources produced a draft
International Code of Conduct for Plant
Germplasm Collecting and Transfer (FAO,
1991).27 This was a voluntary
code of conduct recognizing farmers’
rights and setting guidelines for the
exchange of germplasm. It was addressed
to FAO, UNEP, UNESCO, IBPGR, CGIAR, and
other international and national
agricultural research institutions. In
1992, these issues were highlighted at
the UNCED meeting in Rio de Janeiro, and
through the Global Biodiversity Strategy
(World Resources Institute, 1992).70
These developments have
emphasized the need for researchers,
collectors, and sponsoring organizations
to clarify their codes of ethics. They
have also focused attention on the
importance of developing equitable
partnerships for «capturing» and
effectively dispersing benefits from
these resources, rather than considering
them global commodities. Three features
highlight this issue:
- First, countries
rich in biodiversity generally
have low per-capita income, while
most countries in the North are
wealthy (see Table 2).
- Second, most
companies involved in the
development of new drugs from
plants, micro-organisms, and
marine organisms are based in the
industrialized countries of the
North.
- Third, there is
wide recognition of the role that
economics can play in justifying
conservation as a form of land
use (McNeely, 1988)49
and the need to maximize
"value-adding" from
resource harvesting, including
the use of genes and chemical
structures.
Table 2. Wild plant
diversity and financial income.
Richest in Plant
Diversity
|
N° Plant Species
|
Income/Capita (US$)
|
Richest in Financial
income
|
N° Plant Species
|
Income/Capita (US$)
|
| Brazil |
55,000
|
2,550
|
Switzerland |
2,700
|
30,270
|
| Columbia |
45,000
|
1,190
|
Luxembourg |
1,200
|
24,860
|
| China |
30,000
|
360
|
Japan |
4,040
|
23,730
|
| Venezuela |
25,000
|
2,450
|
Finland |
1,100
|
22,060
|
| South
Africa |
23,000
|
2,460
|
Norway |
1,700
|
21,850
|
| Former USSR |
21,000
|
9,211
|
Sweden |
1,700
|
21,710
|
| Indonesia |
20,000
|
490
|
Iceland |
500
|
21,240
|
| Peru |
20,000
|
1,090
|
USA |
20,000
|
21,100
|
| Mexico |
20,000
|
1,090
|
Germany
(Fed Rep) |
2,480
|
20,750
|
From Davis et al.,
1986; WRI, 1992 22,70
2.Objectives
This paper is intended
for policy makers in governments,
research institutes, botanical gardens,
herbaria, universities, and industry. It
outlines some of the dilemmas facing
ethnobotanists, anthropologists, and
phytochemists in developing new natural
products from biological materials, and
suggests solutions. Recommendations for a
code of practice are made in the
Appendix.
The specific objectives
of this paper are:
- to present the
background to the ethical and
conservation issues that arise in
the development of new natural
products; in particular, to
outline the need to create
equitable partnerships and
recognize the value of indigenous
knowledge which will lead to the
payment of fair compensation to
source regions
- to facilitate
international cooperation in the
collection, conservation, use,
and development of new natural
products
- to ensure that any
collecting for export and use
outside a country has the full
approval of the competent
authorities, and is carried out
with the cooperation of the host
country and representatives of
the local communities involved;
also to ensure that these
collections comply with
conservation and quarantine
regulations in the countries of
origin and destination
- to outline the
general principles that will
facilitate development of
national regulations by
governments or agreements between
organizations.
-
3.
Procedures and Key Players in Chemical
Prospecting
The interest in new
biological compounds has stimulated a
search for sample material from almost
the entire range of life forms, and in
every corner of the globe. Typical
examples include parasitologists working
with Antibody Systems, Inc. searching for
parasites on Tasmanian devils (Bowie,
1992),12 marine biologists
screening Gorgonian corals from tropical
reefs for anti-inflammatory compounds,
botanists collecting medicinal plants for
anti-cancer or anti-HIV activity, and
mycologists collecting soil from tropical
rainforests to find new antibiotics.
The search for new
natural products is widely recognized as
an interdisciplinary process with the
following steps (Hamburger et al.,
1991):33
§ collection, scientific
identification and preservation of the
biological material
§ preparation of appropriate
extracts and preliminary chromatographic
analysis
§ biological and pharmacological
screening of crude extracts
- consecutive steps
of chromatographic separation,
with bioassays for each fraction
(activity- guided fractionation)
§ verification of the purity of
isolated compounds
§ elucidation of structure by
chemical and physico-chemical methods
§ partial or total synthesis
§ preparation of
derivatives/analogues and investigation
of structure-activity relationships
§ large-scale isolation for
further pharmacological and toxicological
tests.
What may be forgotten
in the laboratory is the role that
indigenous knowledge often plays in new
drug discovery (Figure 1). Collectors of
plants for screening take three basic
approaches to obtaining samples (Figure
2).
First, with random
sampling, material is collected from the
largest possible number of identifiable
plant species within a habitat, with the
emphasis on plants that are flowering or
fruiting, so that good voucher specimens
are obtained. A second approach is to
focus collecting on certain plant
families that are known to be rich
sources of interesting, biologically
active compounds such as the Apocynaceae,
Euphorbiaceae, Menispermaceae
, and Solanaceae . Third,
collecting is guided by a knowledge of
traditional uses of plants; this has been
termed «ethnodirected» sampling
(Balick, 1990).8
 |
| Figure 1.
As experts on
«underground botany»
and the symbolic and
active ingredients of
local plants, traditional
healers such as this
diviner (isangoma) in
southern Africa can play
an important role in
«ethno-directed»
sampling. Photo:
A B Cunningham
|
|
Collectors
initially take samples ranging
from 50g for soils to a few
kilograms in the case of plants
or coral. Extracts are prepared
and screened for activity against
diseases such as cancer and AIDS.
In many cases, additional
collections are required, to
obtain sufficient material for
isolation and identification of
the active compound(s). In this
case, large quantities of
material may be collected by
professional contract plant
collectors on short collecting
trips, gathering large bulk
samples for screening. In some
cases, this swift bulk collection
is made possible because of
reference to herbarium material
and ethnobotanical information
previously collected by other
researchers. In the 1970s for
example, in only 17 days in
Tanzania, a USA-based
professional collector gathered
150-kg samples of each of 14
plant species, including 150-kg
samples of bark from Garcinia
smeathmannii and Podocarpus
milanjianus trees. Other
examples are his collections of
10-15 tonnes of Maytenus
buchanani in East Africa and
5 tonnes of Bouvardia
ternifolia plants in Mexico.
This collector still operates
under contract for the American
NCI and has formed an
organization which specializes in
bulk collecting. The sustainability of
this scale of collecting is
questionable. In Kenya, local
overexploitation of the
widespread shrub Maytenus
buchanani certainly took
place in a conservation area in
the Shimba hulls so that NCI
could screen for its value as an
anticancer agent. When additional
material was required four years
after the first harvesting in
1972, regeneration was found to
have been so poor that collectors
reportedly struggled to obtain
additional material (Juma, 1989).40
Fortunately, the recent surge of
interest in screening for new
compounds has increased awareness
of the conservation implications
of destructive harvesting and the
need for equitable partnerships.
|
| |
|
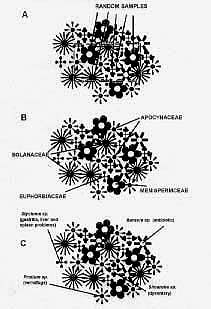 |
Figure 2. The
three main collection methods
used in sampling plant material
for biologically active compounds
(Balick, 1990).7 A:
Random sampling. B: Taxonomically
directed sampling. C:
«pre-screening» based on
indigenous knowledge of plant
uses. This has been termed
«ethno-directed» sampling. |
| |
|
 |
Figure 3. Fruits
and seeds of Castanospermum
australe (Fabaceae). This
Australian tree is the source of
the anti-viral compound,
castanospermine, which has been
used in treating the HIV virus
which causes AIDS. Photo A.B.
Cunningham |
Table
3. Pharmaceutical companies and research
organizations involved in screening
plants for new natural products, showing
sources of supply.
| Organization |
Status of
Plant Screening Programme |
Supplied by |
Region of
Origin |
| American
National Cancer Institute (NCI) |
Large scale
screening of plants, also marine
organisms |
Missouri
Botanical Garden, New York
Botanical Garden, University of
Illinois, private contractors |
Africa,
Madagascar, Central and South
America, Southeast Asia,
Australia |
| Bristol-Meyers |
None at
present. Evaluating whether to
include plants or not. Developed
taxol from Pacific yew (Taxus) |
Not
applicable |
Taxol
material from USA |
| Glaxo |
Natural
products discovery department.
Many therapeutic areas |
Commercial
and academic institutions. Royal
Botanic Gardens, Kew |
South
America, Africa |
| Merck,
Sharp Dohme Research Laboratories |
Marine
organisms, plants and
micro-organisms |
Kew New
York Botanical Garden. Work with
INBio, Costa Rica |
South
America |
| Monsanto/Searle |
Micro-Organisms
and plants |
Missouri
Botanical Garden |
North
America |
| Shaman
Pharmaceuticals |
Plants
based on ethnobotanical
information |
Individuals,
institutions and government
departments |
Tropical
South America, Africa, Southeast
Asia |
| SmithKline
Beecham |
Marine
organisms, plants and
micro-organisms |
Biotics
Ltd, private individuals and own
collectors |
Malaysia,
Micronesia |
From Findeisen, 199129
and other sources
Recent advances in
molecular and biochemical pharmacology
have made it easier to carry out new
assays for the development of drugs from
plants, micro-organisms, and marine
corals. As a result, new screening
programmes have been started in a wide
range of developing countries. These
programmes focus on identifying active
compounds with anti-cancer, anti-HIV,
anti-inflammatory, antibiotic, or
anti-parasitic activity. Endemic species
are of particular interest. There is also
a resurgence of interest in soil fungi,
particularly non-streptomycetous
actinomycetes, due to increasing
resistance in humans to commonly used
antibiotics.
The value of contracts
awarded by the US Developmental
Therapeutics Programme (DTP) of the NCI
indicates how much interest there is in
discovering new natural-product drugs. In
1986 three five-year contracts worth $2.7
million were awarded. In 1991 these were
renewed, with the three contracts valued
at $3.8 million (NCI, 1992).51
The three contractors are all based in
the USA: Missouri Botanical Garden,
Bishop Museum, Honolulu, and New York
Botanical Garden together with the
University of Illinois at Chicago, which
subcontracts to the Arnold Arboretum
(Harvard University). Collections are
focused respectively on Africa and
Madagascar, Central and South America,
and Southeast Asia. These collaborative
programmes have resulted in the discovery
of several interesting new compounds,
including some with anti-HIV properties,
from the Ancistrocladaceae,
Combretaceae, Euphorbiaceae, and Piperaceae
(Kashman et al., 1992;41 Table
4). A similar five-year contract, valued
at $2.9 million, was awarded to the Coral
Reef Foundation for collection of marine
organisms to determine their potential as
the source of valuable new compounds.
In the past, collecting
has been largely uncontrolled, with
sample material usually being taken for
analysis to Europe, Japan, or North
America. Drug development and patenting
has taken place without the knowledge of
people in the country of origin, with no
recompense for use of regional natural
resources, and often without any
contractual obligation. When permit
applications have been made, this has
been done without indicating the
commercial intent of the collectors.
Local professionals such as botanists or
foresters are paid privately to collect
samples for industrial companies or other
sponsoring organizations. These people
often do not understand the full
implications of their work and their
payment bears little relation to the
potential value of the resource. As many
developing countries pay them relatively
little, and hard currency is hard to get,
it is understandable that this occurs.
Nevertheless, it has important
implications for regional development and
conservation. From the point of view of
national and local interests, why should
these sponsoring organizations be allowed
to treat a county's natural resources as
global common property, especially
considering that the natural resources
may later be privatized by the same
organization?
Table 4. Examples of
drugs and new therapeutic agents
discovered by screening plants for
biologically active substances.
(Also indicated:
species’ region of origin, potential
clinical use, and use in traditional
medicine)
| Species |
Family |
Origin |
Clinical
Use |
Trad.
Medicine |
Developer |
| Ancistrocladus
abbreviatus |
Ancistrocladaceae |
Cameroon |
anti-viral
(HIV) |
no related
use recorded |
USA (NCI) |
| Ancistrocladus
sp. nov. |
Ancistrocladaceae |
Cameroon |
anti-viral
(HIV) |
no related
use recorded |
USA (NCI) |
| Artemisia
annua |
Asteraceae |
China |
anti-malarial |
anti-malarial |
China,
Myanmar |
| Calophyllum
lanigerum |
Clisiaceae |
Malaysia |
anti-viral
(HIV) |
? |
USA (NCI) |
| Camptotheca
acuminata |
Nyssaceae |
China |
anti-cancer |
? |
China, USA |
| Castanospermum
australe |
Fabaceae |
Australia |
anti-viral
(HIV) |
no related
use recorded |
USA (NCI) |
| Catharanthus
roseus |
Apocynaceae |
Madagascar |
anti-cancer |
anorexigenic
(?), random screen |
USA
(Eli-Lilly) |
| Cephalotaxus
harringtonia |
Cephalotaxaceae |
China |
anti-cancer |
? |
China, USA
(NCI) |
| Cinchona
pubescens |
Rubiaceae |
South
America |
anti-malarial |
anti-malarial |
Europe |
| Coleus
forskolii |
Lamiaceae |
India,
Sri-Lanka, East Africa |
anti-metastatic |
cardio-vascular,
respiratory and renal use |
India /
Germany (Hoechst) |
| Ginkgo
biloba |
Ginkgoaceae |
China |
anti-asthmatic
also evaluated graft rejection |
anti-asthmatic |
France
(Beaufour labs), Germany
(Schwabe) |
| Homalanthus
nutans |
Euphorbiaceae |
Samoa |
anti-viral
(VIH) |
anti-diarrhoeal |
USA (NCI) |
| Ochrosia
elliptica |
Apocynaceae |
Pacific |
anti-cancer |
? |
France
(Sanofi) |
| Piper
futokadsura |
Piperaceae |
China |
bronchoasthma |
bronchoasthma,
stiffness |
USA (Merck,
Sharp & Dohme) |
| Podophyllum
peltatum |
Berberidaceae |
North
America |
anti-cancer |
cathartic,
anthelimintic |
USA (NCI)
Switzerland (Sandoz) |
| Taxus
brevifolia |
Taxaceae |
North
America |
anti-cancer |
known to be
highly toxic |
USA (NCI)
and Bristol-Meyers-Squibb |
| Trichosanthes
kirilowii |
Curcurbitaceae |
China |
anti-viral
(HIV) |
? |
USA |
From Hamburger et al.,
1991;33 Manfredi et al.,
1991;47 Kashman et al.,
1992;41 Gustafson et al.,
1992.32
NEXT
|