Abstract
The microrelief of plant surfaces, mainly
caused by epicuticular wax crystalloids, serves different purposes and
often causes effective water repellency. Furthermore, the adhesion of contaminating
particles is reduced. Based on experimental data carried out on microscopically
smooth (Fagus sylvatica L., Gnetum gnemon L., Heliconia
densiflora Verlot, Magnolia grandiflora L.) and rough water
repellent plants (Brassica oleracea L., Colocasia esculenta
(L.) Schott., Mutisia decurrens Cav., Nelumbo nucifera Gaertn.),
it is shown here for the first time that the interdependence between surface
roughness, reduced particle adhesion and water repellency is the keystone
in the self-cleaning mechanism of many biological surfaces. The plants
were artificially contaminated with various particles and subsequently
subjected to artificial rinsing by sprinkler or fog generator. In the case
of water repellent leaves, the particles were removed completely by water
droplets that rolled off the surfaces independent of their chemical nature
or size. The leaves of N. nucifera afford an impressive demonstration
of this effect, which is, therefore, called the "Lotus-Effect" and which
may be of great biological and technological importance.
Keywords: Contamination, Cuticle, Epicuticular
wax, Lotus-effect, Nelumbo, Wettability
Introduction
All primary parts of plants (except roots)
are covered by a cuticle that is the interface between plants and their
environment. The cuticle is composed of soluble lipids embedded in a polyester
matrix (Holloway 1994). Due to its chemical composition, the cuticle in
most cases forms a hydrophobic surface.
In the past 25 years, scanning electron
microscope studies of biological surfaces have revealed an incredible microstructural
diversity of the outer surfaces of plants. Microstructures such as trichomes,
cuticular folds, and wax crystalloids serve different purposes and often
provide a water repellent surface, which is not rare in terrestrial plants.
Water repellency is mainly caused by epicuticular wax crystalloids which
cover the cuticular surface in a regular microrelief of about 1-5 µm
in height (Jeffree 1986).
The principle connections between surface
roughness and water repellency were worked out by Cassie and Baxter (1944).
Later, the wetting properties of surfaces were the subject of intensive
studies in physics as well as in biology and reviewed several times (e.g.
Adamson 1990; de Gennes 1985; Fowkes 1964; Holloway 1970).
In addition, the measurement and characterization
of different kinds of particles and their impact on vegetation has been
studied thoroughly (e.g. Belot and Gauthier 1975; Chamberlain and Little
1981; Coe and Lindberg 1987; Farmer 1993; Little 1979).
The relationship between surface roughness
and wettability or particle deposition, respectively, is well known. Surprisingly
few reports exist - most of which lack experimental data - concerning the
correlation between surface roughness, water repellency and the removal
of particles (Barthlott 1990; Davies 1961; Rentschler 1971). However, there
has been a vague knowledge of the correlation between water repellency
and reduced contamination for more than a century (Lundström 1884).
During the routine interpretation with
respect to systematics of SEM micrographs of the leaf surfaces of some
10,000 plant species (Barthlott 1990; Barthlott 1993), we observed a peculiar
effect. Independently of the degree of pollution at the collection site,
species with smooth leaf surfaces always had to be cleaned before examination,
while those with epicuticular wax crystals were almost completely free
of contamination.
Later, we validated these observations
by performing simple qualitative experiments. As one example, microscope
slides were affixed to water repellent leaves of Indian cress (Tropaeolum
majus) and examined in the SEM six weeks later. While the rough leaf
surface was virtually clean, the smooth glass slides had accumulated particulate
contaminations. Based on a number of experiments, it was proven that water
repellency causes an almost complete surface purification (self-cleaning
effect): contaminating particles are picked up by water droplets or they
adhere to the surface of the droplets and are then removed with the droplets
as they roll off the leaves.
Material and Methods
Plant material. Leaves from approximately 340 plant species, cultivated at the Botanical Garden in Bonn, were investigated by contact angle measurements and high resolution scanning electron microscopy (SEM). A selection of these species was used for quantitative contamination experiments. Smooth surfaces of differing wettability (Table 1) were represented by the leaves of two evergreen trees (Gnetum gnemon L., Magnolia denudata L.), the rainforest herb Heliconia densiflora Verlot, and beech (Fagus sylvatica L.). Water repellent, rough surfaces were represented by leaves of the sacred lotus (Nelumbo nucifera Gaertn.), kohlrabi (Brassica oleracea L.), taro (Colocasia esculenta (L.) Schott.) and the petals of a composite (Mutisia decurrens Cav.).
Contact angle (CA) measurement. Measurement of CA were carried out on the adaxial surfaces of young, fully developed leaves. Samples of 1 cm2 cut from the central area of the leaf lamina were affixed to glass slides by double-sided adhesive tape (TesaFix, Beiersdorf, Hamburg, Germany) to ensure an even surface. Aqua dest. droplets of 10 µl were applied to the surface and the static contact angle was measured with a horizontal microscope equipped with the Goniometer G1 (Erma Optical, Tokyo, Japan). In Table 1, the mean values and standard deviation of 20 contact angle measurements are noted.
| Heliconia densiflora | 28.4 ± 4.3 |
| Gnetum gnemon | 55.4 ± 2.7 |
| Magnolia denudata | 88.9 ± 6.9 |
| Fagus sylvatica | 71.7 ± 8.8 |
| Nelumbo nucifera | 160.4 ± 0.7 |
| Colocasia esculenta | 159.7 ± 1.4 |
| Brassica oleracea | 160.3 ± 0.8 |
| Mutisia decurrens | 128.4 ± 3.6 |
Table 1. Mean values (±SD) of 20 measurements of the static contact angle (°) on the adaxial leaf surfaces of the species used for contamination experiments
Scanning electron microscopy (SEM). For examination of the surface relief, samples of about 1 cm2 cut from the middle of the leaf lamina of young, fully developed leaves were prepared by liquid substitution according to Ensikat and Barthlott (1993). For high resolution SEM of epicuticular waxes, fresh leaf samples were affixed to aluminium stubs by doublesided adhesive tape (TesaFix) and air dried. All specimens were sputter coated (Balzers Union SCD 034, Balzers, Wiesbaden) and examined in a Cambridge Stereoscan 200 (Leica, Bensheim, Germany) equipped with a Lanthan-Hexaboride-cathode.
Contaminants. Several different particles of various grain sizes (range of the diameters is given) were used for contamination: dried soil, barium sulphate (5-30 µm), siliciumcarbide dust 360 (5-25 µm) and 1200 (0,5-3,5 µm, both Guilleaume, Bonn, Germany), titan dioxide (1 - 20 µm; Merck, Darmstadt, Germany), toner of a Xerox photocopier (5 µm), Sudan-III pigment powder (1 - 20 µm, Merck), silanized (Silbond 600 MST) and non-silanized (Silbond 600 AST) quartz dusts (6-25 µm, Quarzwerke, Frechen, Germany), spores of the tree fern Cibotium schiedei (20 µm), and conidia of the grey mould Botrytis cinerea (20 - 30 µm) and Tiletia caries (20 µm).
Artificial contamination. Whole plants or individual leaves were placed in a contamination chamber consisting of a frame measuring 60x60x100cm and covered by plastic foil. The chamber was divided by a removable wooden plate 40 cm above the ground covering the specimens. The contaminants were blown into the upper part of the contamination chamber by pressured air. After 15 s, when the largest particles and particle aggregates had sedimented onto the plate, the latter was removed and the particles were able to deposit onto the specimens evenly. Particle density could be varied by the time of exposure to the dust. After each contamination, the chamber was cleaned with water. The number of particles was determined by SEM in combination with a digital image analysis system (TCL-Image, Multihouse TSI, Amsterdam, The Netherlands) before and after rinsing.
Artificial rinsing. Following contamination, the specimens were subjected to natural and artificial rain of various droplet sizes. Artificial rain was simulated by a sprinkler producing droplets of 0.5-3 mm diameter. Fog treatment was performed in a chamber equipped with a high pressure fog system (Osberma, Engelskirchen, Germany) producing very fine fog droplets (1-20 µm diameter). Fog treatment was performed in order to obtain an experimental set-up which avoided the high kinetic energy of larger water droplets.
Results
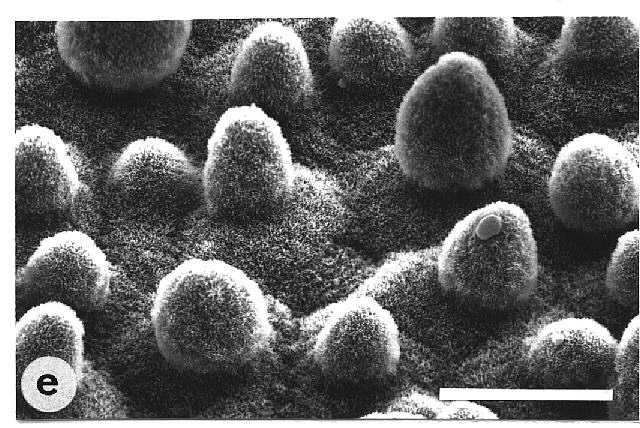
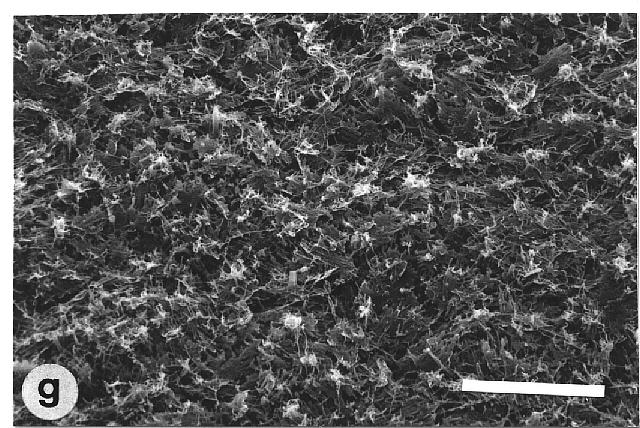
Surface characteristics and wettability
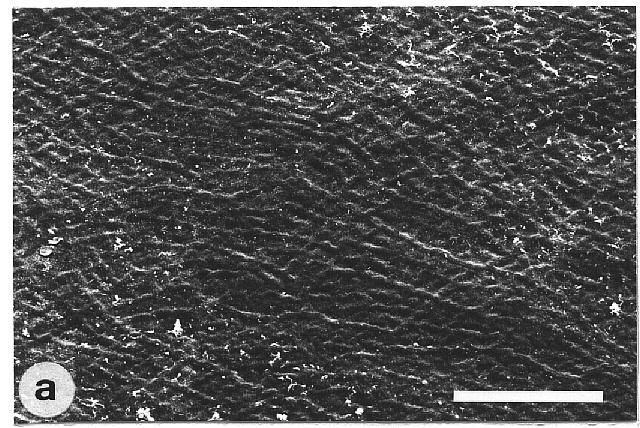
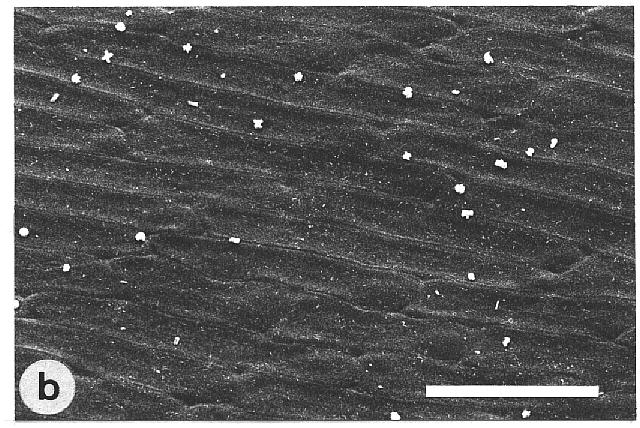
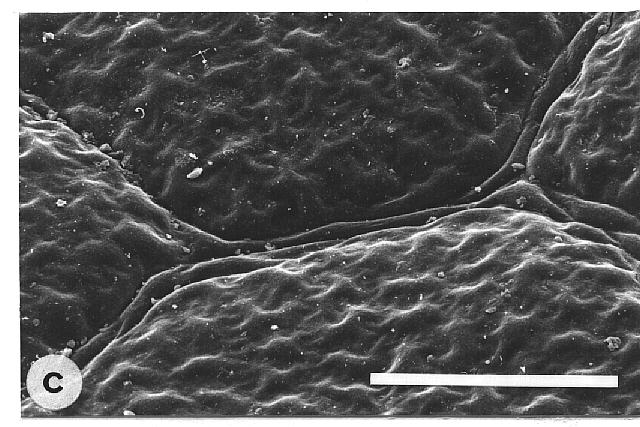
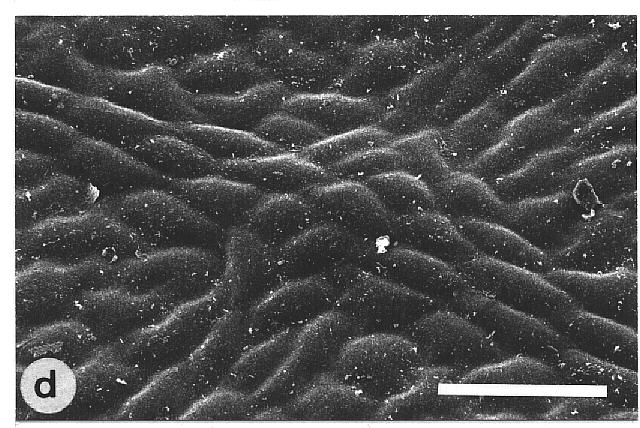
of microscopically rough and smooth leaves. The majority of the wettable
leaves (CA <110°) investigated were ± smooth, without any
prominent surface sculpturing. In particular, epicuticular wax crystals
were absent (Fig. 1, a-d). Some leaves were covered by trichomes during
leaf expansion and several species showed an ornamentation due to slightly
convex epidermal cells, as well as sunken or raised nerves. The contact
angles ranged from 110° in young to less than 10° in old, naturally
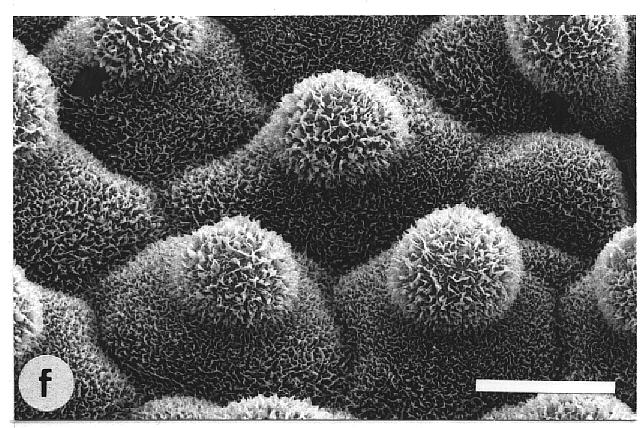
contaminated leaves. In contrast, water repellent leaves exhibited various
surface sculptures, mainly epicuticular wax crystals in combination with
papillose epidermal cells (Fig. 1, e-g). Their contact angles always exceeded
150°. The petals of Mutisia decurrens were characterized by
cuticular folds (fig. 1h). They were not as water repellent as leaves with
epicuticular wax crystalloids, but they had higher mean contact angles
than smooth leaves.
Depending on the wettability, applied
water droplets (40 µl) displayed distinct characteristics when rolling
off the leaves. In water repellent surfaces, water contracted to form spherical
droplets. It ran off the leaf very quickly even at slight angles of inclination
(<5°) without leaving any residue. In smooth leaves with contact
angles above 70°, water contracted to form ± hemispherical droplets
that ran off the leaves comparatively slowly and at higher angles of inclination
(10-30°). This was true also for the petals. In smooth leaves with
low contact angles, water spread quickly and partially ran off the leaves
at inclination angles of >40°.
 |
 |
 |
 |
 |
 |
 |
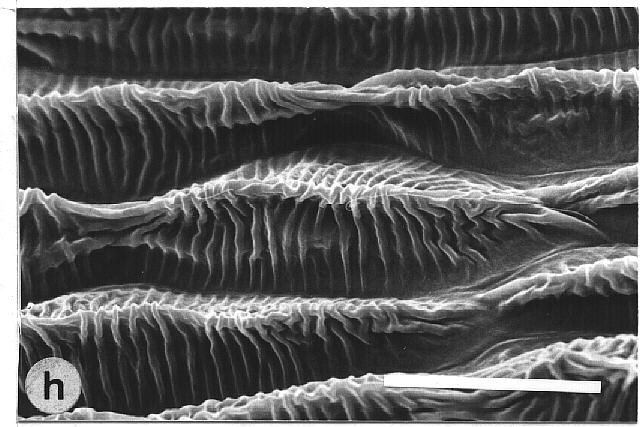 |
Fig. 1a-h. Scanning electron micrographs of the adaxial leaf surface of smooth, wettable (a-d) and rough, water repellent (e-h) leaf surfaces. The smooth leaves of Gnetum gnemon (a) and Heliconia densiflora (b) are almost completely lacking microstructures while those of Fagus sylvatica (c) and Magnolia denudata (d) are characterized by sunken and raised nervature, respectively. The rough surfaces of Nelumbo nucifera (e) and Colocasia esculenta (f) are characterized by papillose epidermal cells and an additional layer of epicuticular waxes. Brassica oleraces leaves (g) are densely covered by wax crystalloids without being papillose, and the petal surfaces of Mutisia decurrens (h) are characterized by cuticular folds. Bars: 100 µm (a-d) and 20 µm (e-h). |
|
The self-cleaning properties of microscopically
rough and smooth surfaces. In general, particles of any kind were always
removed entirely from water repellent leaves when subjected to natural
or artificial rain, independent of their size and chemical nature, as long
as the surface waxes were not destroyed. Fig. 2 summarizes four series
of experiments carried out with Sudan III, bariumsulfate, spores of Cibotium
schiedei, and conidia of Botrytis cinerea. After contamination,
the leaves were subjected to artificial rain for 2 min. at an inclination
angle of 15°. While the smooth surfaces retained 40 - 80% of their
particles after the treatment, the sculptured surfaces were completely
cleaned. Although the contact angles were considerably lower in M. decurrens
petals, they also displayed a high self-cleaning ability.
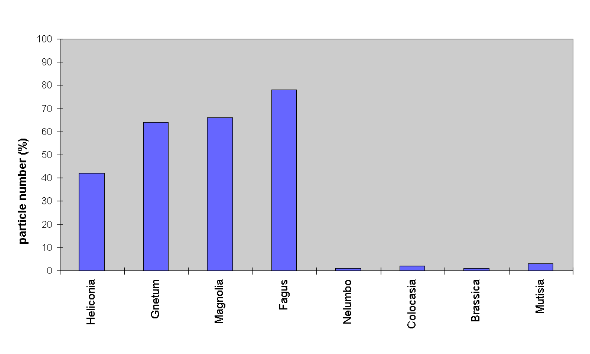 |
Fig. 2. Results summarizing four series of contamination experiments
carried out with Sudan III, bariumsulfate, Cibotium schiedei spores
and Botrytis cinerea conidia. The columns represent the mean values
of the percentage of particles remaining on smooth and rough leaves after
artificial rinsing; initial number set as 100%.
|
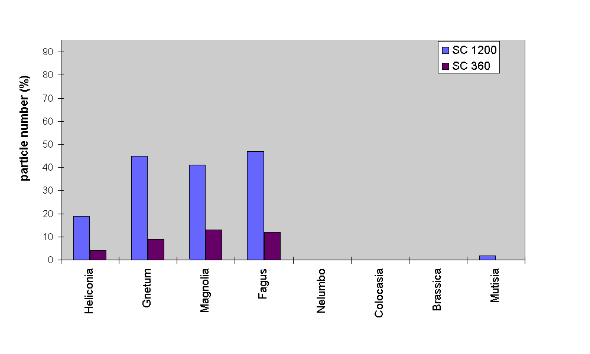 |
Fig. 3. Particles remaining on smooth and rough leaf surfaces
after artificial contamination with siliciumcarbide of two different grain
sizes and exposure to a natural rain storm. While rough surfaces are completely
cleaned, smooth surfaces retain 5-50 % of the particles, depending on the
particle size.
|
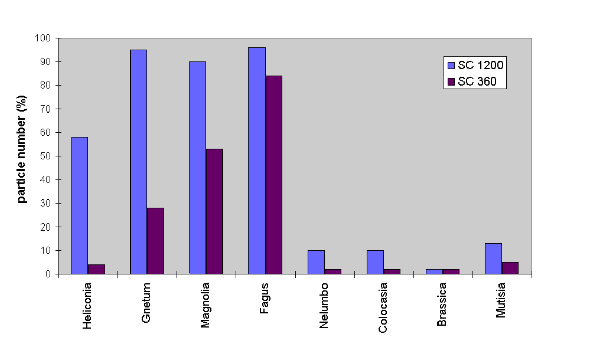 |
Fig. 4. Particles remaining after artificial contamination with siliciumcarbide 360 and 1200, subjected to 1 mm artificial fog at 15° inclination angle. Among species with water repellent surfaces the ones with epidermal papillae retain a small amount of very fine particles. |
Generally, the results were not influenced by particle chemistry and size as well as procedure and duration of rinsing. Wettable plant surfaces always retained a considerable number of the contaminating particles after rinsing and drying of the leaves. Larger numbers of particles were removed from smooth leaves only when subjected to heavy natural rain. Especially droplets of rainstroms are very large and have a high kinetic energy which facilitates particle removal. Figure 3 presents the amount of particles remaining after contamination with SC 1200 and 360 and exposure to a natural rainstorm of a total precipitation amount of 5 mm.
When subjected to very fine fog having
droplets of almost no kinetic energy, the results were different. The rough
surfaces of N. nucifera and C. esculenta retained a certain
amount of extremely small particles (e.g. SC 1200) on the leaf within the
troughs between the epidermal papillae (Fig. 4). However, these particles
were easily removed from the surfaces when subjected to gentle rain or
to single droplets that were dropped onto the leaves from a height of about
5 cm.
The effect can be demonstrated on the
microscopic level using mercury as an anlogous liquid. The roughness of
the papillose leaves leads to a reduced contact area between particles
and surface (fig. 5) as well as between droplets and surface (Fig. 6).
The droplet rests only on the tips of epicuticular wax crystals on the
top of the papillose epidermal cells. Contaminating particles are picked
up by the liquid and carried away while the droplet rolls off the leaf
(Fig. 7).
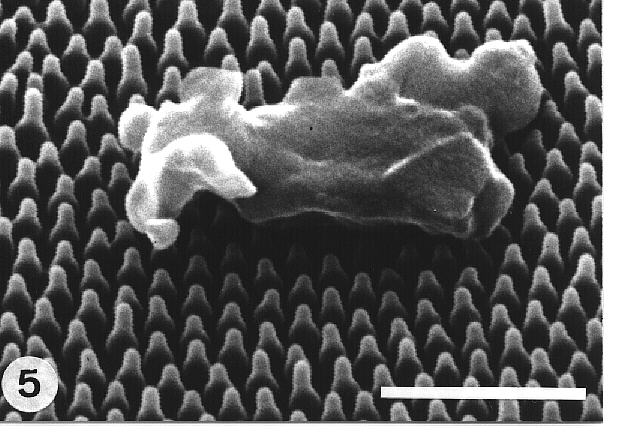 |
Fig. 5. Contaminating particle on a regularly sculptured wing surface of Cicada orni, demonstrating the decreased contact area between a particle and a rough surface. Bar: 1µm. |
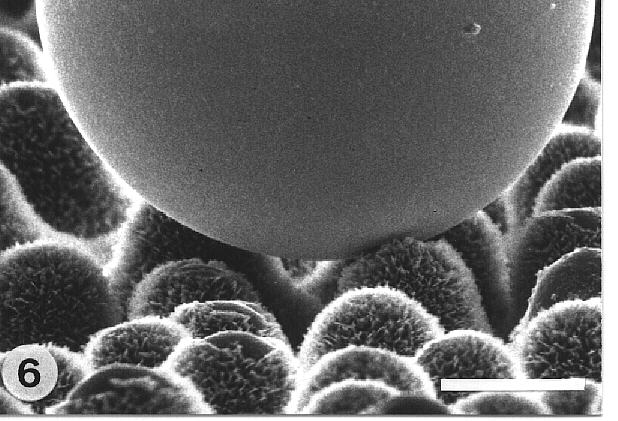 |
Fig. 6. Mercury droplet on the papillose adaxial epidermal surface of Colocasia esculenta demonstrating the effect of roughness on wettability. Due to the decreased contact area between liquid and surface, air is enclosed between the droplet and the leaf resulting in a particularly strong water repellent surface. Bar: 20 µm. |
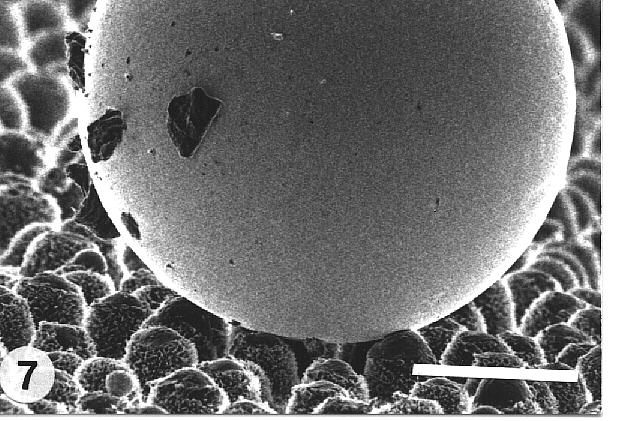 |
Fig. 7. Mercury droplet on the adaxial leaf surface of Colocasia esculenta demonstrating the Lotus-Effect. Contaminating particles adhere to the surface of the droplet and are removed from the leaf while the droplet rolls off. Bar: 50 µm. |
Discussion
The results presented above document an almost complete self-cleaning ability by water repellent plant surfaces. This can be demonstrated most impressively with the large peltate leaves of the sacred lotus (Nelumbo nucifera). According to tradition in Asian religions, the sacred lotus is a symbol for purity, ensuing from the same observations we have made. This knowledge is already documented in Sanskrit writings, which fact has led us to call this phenomenon the "Lotus-Effect".
Physical background of the Lotus-Effect.
The surface physics behind the Lotus-Effect can be derived from the
behavior of liquids applied to solid surfaces. Up to present, there are
only a few investigations dealing with the interaction between rough biological
surfaces, particles and water. However, since the wettability of solid
surfaces is well investigated in surface science (e.g. Dettre and Johnson
1964; de Gennes 1985; Adamson 1990; Myers 1991), it is possible to draw
conclusions about the conditions on leaf surfaces.
The wetting of a solid with water, with
air as the surrounding medium, is dependent on the relation between the
interfacial tensions (g)
water/air (gwa),
water/solid (gws)
and solid/air (gsa).
The ratio between these tensions determines the contact angle q
of a water droplet on a given surface and is described by Young`s equation
gsa
- gws
= gwa
cosq.
A contact angle of 0° means complete wetting, and a contact angle of
180° corresponds to complete non-wetting. Neither case is apparent
in plant cuticles. Solids with large gsa
are more easily wetted compared to those with low gsa
(e.g. Teflon). In the latter, water tends to form hemispherical droplets
with a high contact angle.
If a droplet is applied to a solid surface,
it will wet the surface to a certain degree. The amount of wetting depends
on the ratio between the energy necessary for the enlargement of the surface
and the gain of energy due to adsorption, which compensates for the former.
At equilibrium, the energy of the system is minimized (Adamson 1990, Myers
1991).
Surfaces with only few or completely lacking polar groups exhibit a
very low interfacial tension (de Gennes 1985). This applies also to many
components of epicuticular waxes (e.g. hydrocarbons). In the case of water
repellent rough surfaces, air is enclosed between the epicuticular wax
crystalloids, forming a composite surface (Fig. 6). This enlarges the water/air
interface while the solid/water interface is minimized (Dettre and Johnson
1964; Holloway 1970). On such a rough "low energy" surface, the water gains
very little energy through adsorption to compensate for any enlargement
of its surface. In this situation, spreading does not occur, the water
forms a spherical droplet, and the contact angle of the droplet depends
almost entirely on the surface tension of the water.
Particles deposited on a waxy surface consist, in most cases, of material
which is more readily wetted than hydrophobic wax components. In addition,
they are in general larger than the surface microstructures and rest only
on the very tips of the latter (Fig. 5). As a result, the interfacial area
between both is minimized. In the case of a water droplet rolling over
a particle, the surface area of the droplet exposed to air is reduced
and energy through adsorption is gained. The particle is removed from the
surface of the droplet only if a stronger force overcomes the adhesion
between particle and water droplet (Adamson 1990). On a given surface,
this is the case if the adhesion between particle and surface is greater
than the adhesion between particle and water droplet. Due to the very small
interfacial area between particle and rough surface, adhesion is minimized.
Therefore the particle is "captured" by the water droplet and removed from
the leaf surface (Fig. 7).
The effectiveness of the self-cleaning ability decreased in the case
of fog and dew, in contrast to rain. Rain droplets have a high kinetic
energy. Elastic deformation allows them to penetrate between epidermal
papillae and remove particles within the troughs. This does not
happen with fog, and especially not with dew. This explains the fact that
in the case of the papillose leaves of N. nucifera and C. esculenta,
a certain amount of very small particles is retained after fog treatment,
while no difference could be observed in the non-papillose leaves of B.
oleracea.
The quantity of particles removed from a smooth surface depends mainly
on its wettability. In surfaces with high contact angles, spreading is
very limited, and the velocity of droplets running off a surface is relatively
low. Therefore, particles are mainly displaced to the sides of the droplet
and redeposited behind the droplet, but not removed. Especially hydrophobic
particles tend to remain on such surfaces. This result, which was observed
also by Davies (1961), may be explained by the similar interfacial tensions
between the particles and the surface. In surfaces with a low contact angle,
droplets spread very quickly and the water runs off the leaves with considerable
velocity. It is very likely that particles are carried along with the moving
liquid front, a mechanism that was also presumed responsible for the removal
of microorganisms from leaf surfaces (Lips and Jessup 1979). This explains
the comparatively effective cleaning effect in the leaves of Heliconia,
in contrast to Magnolia and Gnetum. After several weeks,
however, Heliconia leaves also accumulate particles and are easily
colonized by bacteria, fungi, and algae, which was not observed in water
repellent leaves. The latter always exhibited a very low degree of contamination.
In intact leaves, a colonization with bacteria or algae could not be observed.
The disparate results obtained from smooth and rough surfaces with
respect to wettability and particle removal are summarized in Fig. 8 a,b.
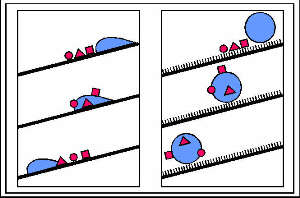 |
Fig. 8. The drawing summarizes the connection between roughening and self-cleaning. While in smooth surfaces the particles are mainly redistributed by water (a), they adhere to the droplets surfaces in rough surfaces and are removed from the leaves when the droplets roll off (b). |
The biological implications of the Lotus-Effect. The cuticle
is the outermost barrier of plants towards their environment and is, therefore,
the first protective layer (Dickinson 1960; Martin 1964; Campbell et al.
1980; Juniper 1991). Because the air contains many kinds of particles,
leaf surfaces are continuously contaminated. Many deposits are ±
neutral, but various kinds of contamination may cause considerable damage
to the plants, depending on size and chemical nature. It was shown that
in polluted areas where plants are heavily contaminated with dust, leaf
surface temperatures increased under insolation (Eller 1977). In addition,
particles within a certain size range may occlude stomata and influence
stomatal diffusive resistance (Flckiger et al. 1979). These and other
interactions between dust particles and plants have been reviewed extensively
by Farmer (1993). Water repellent plants escape from those harmful effects
through Lotus-Effect. Although it was shown that particles are captured
more effectively by rough leaf surfaces (Chamberlain 1967; Belot and Gauthier
1975), this disadvantage is compensated for by a very effective self-cleaning
capability.
The Lotus-Effect plays another important role in the defense against
pathogens. Spores and conidia of pathogenic microorganisms, as well as
inorganic particles, are deposited on the leaf surfaces. Again, wettability
is important for the adhesion of microorganisms to leaf surfaces (Rogers
1979). In addition, on water repellent surfaces, spores and conidia are
deprived of the water necessary for germination (Campbell, et al. 1980;
Allen et al. 1991; Juniper 1991). Therefore, the epicuticular wax crystalloids
and their physical properties may be regarded as the first line of defense
against pathogens. Our results indicate that the Lotus-Effect may be the
most important function of epicuticular waxes and the reason for pervasive
microsculpturing of many leaf surfaces. There are but a few pathogens that
are able to overcome this barrier. Powdery mildews, for example, contain
a small amount of water within their conidia which enables them to germinate
on virtually dry surfaces. A dry surface seems to be beneficial to them,
while there is some indication that a wet surface may impede germination
(Wheeler 1981).
As shown before, epicuticular wax crystalloids provide a highly effective
self-cleaning surface to many plants. On the other hand, they are very
fragile structures and may be easily altered, especially by mechanic abrasion
(van Gardingen et al. 1991; Bermadinger-Stabentheiner 1994). This also
influences the water repellent function: within altered areas, particles
may be retained permanently. If the damage is not too grievous,
it can be compensated for by the regeneration of the wax crystalloids (e.g.
Hallam 1970). Apart from naturally occurring wax alterations, some anthropogenic
influences can be very serious. This is especially true for surfactants.
They are an important component in all-water based pesticides, since they
enable the uptake of an active ingredient through the cuticle (Stevens
and Bukovac 1985; Lownds et al. 1987; Knoche and Bukovac 1993). However,
the surfactants cause considerable damage to wax ultrastructure (Noga et
al. 1987; Wolter et al. 1988). Due to the alterations in the wax ultrastructure,
the wettability of the leaves is increased for at least several days and
water is retained within the altered areas. As a result, contaminating
particles including spores and conidia of pathogens are also found within
the areas of altered waxes (Neinhuis 1992). Under unfavourable conditions,
the probability of an infection in a plant may be enhanced, which is in
contrast to the aim of a pesticide application.
The Lotus-Effect is not restricted to plants; indeed, it is of an overall
biological importance, e.g. for insects. Especially those insects with
large wings, which cannot be cleaned by legs, have water repellent wing
surfaces and exhibit the self-cleaning ability (Wagner et al. 1996). In
this case, not only the removal of particles is of interest, but also the
maintenance of flight capability, which may be lost due to an unequal load
on the wings.
Conclusion. Many terrestrial plants and animals are water repellent due to hydrophobic surface components in connection with a microscopic roughness. It was shown that these surfaces provide a very effective anti-adhesive property against particulate contamination. This self-cleaning mechanism, called the Lotus-Effect, may be the most important function of many microstructured biological surfaces. We assume that this effect can be transferred to artificial surfaces (e.g. cars, facades, foils) and thus find innumerable technical applications.
Acknowledgements
The present paper is based on research that was supported by serveral
organisations and persons. For funding we are indebted to: Bundesministerium
für Forschung und Technologie, Deutsche Forschungsgemeinschaft, Akademie
der Wissenschaften und Literatur, Mainz.
For helpful comments and discussions we are indebted to H. Erhard and
co-workers, Kaiserslautern; Z. Hejnowicz, Kattowice, Bonn; M. Markus, Dortmund;
A. Sievers, Bonn.
References