Biological objects can only be examined with the electron microscope after long and careful preparations. The danger of artefacts is readily given. D. W. FAWCETT wrote in 1964:
"It has to be agreed without reservations that we have no objective criteria to judge the good maintenance of the examined structures. Maybe it is more a belief than a proven fact among morphologists that an image that is clear, continuous, well-ordered, detailed and generally aesthetic is more likely to represent reality than one that is rough, unordered and blurred. But to choose any other criteria as a basic principle would mean encouragement of less carefulness and technical bungling."
Years of research and results achieved with different methods
finally guaranteed the reliability of the methods and results.
Biological preparations - even single cells - are usually too big and
too thick to be used as a whole. Normally
cross-sections have to be
prepared. Sections require the following steps:
- Fixation of the material, usually with glutaraldehyde (covalent cross-linking of protein molecules) and osmium tetroxide (binds to and stabilizes membranes). Dehydration of the specimen.
- Permeation with a monomeric resin that polymerizes to form a solid block of plastic. Without the thus strengthened structures the specimen would collapse in the vacuum of the microscope.
- Cutting of the specimen: needed is an ultramicrotome that can produce sections of about 15 - 100 nm thickness (about 1/200 of the thickness of a cell). Ultramicrotomes do normally have fine glass knifes. Edges of break of glass are sharper than metal knifes, but they do not last very long. Diamond knifes are an alternative. They live longer, but are also much more expensive.
- The thin sections are placed on a small circular metal grid that is coated with a coal-strengthened plastic (formvar) for viewing in the microscope.
- Two different methods for contrast enhancement exist: coating and impregnation with heavy metal ions. Coating is achieved by placing the specimen in a vacuum and exposing it to a cloud of metal dust (platinium, platinium/coal, gold, vanadium, chrome, lead, etc.). The cloud is produced by the heating of a metal filament that is placed at a defined distance and angle from the specimen. The relief-like surface of the specimen helps the formation of irregular structured metal coats on the specimen (relief contrast). The imprint of a specimen can also be taken. Therefore slightly thicker metal coats are prepared that are taken off the specimen before viewing them in the microscope.
When contrast is achieved with heavy metal ions, then the preparation is impregnated with uranyl acetate- or lead citrate solutions. The salts are absorbed by the specimen with different strength, so that differently labeled structures are viewed in the microscope later on. It is spoken of positive staining, if a special structure has absorbed the ions and of negative staining, if the metal ions (phosphoric tungsten acid, uranyl acetate, uranyl formiate and others) accumulate around the actual structure. Negative staining is normally used to make macromolecules and molecular complexes (ribosomes, viruses) visible. Usually a special chemical has to be added that prevents the molecules from getting lumpy.
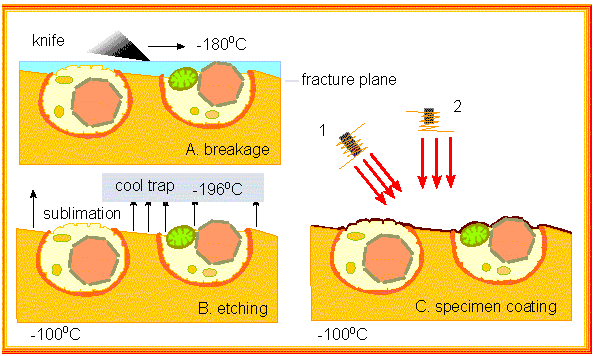
Freeze methods (like freeze-drying) offer an alternative to chemical fixation with often better preservation of the specimen's structures. Freeze-fracture and freeze-etching (H. MOOR and K. MÜHLENTHALER; University of California, Berkeley and Eidgenössische Hochschule Zürich, 1963) are well-suited for small specimen: cells and subcellular structures. The specimen is frozen and the frozen material is "broken" with the aid of a glass knife. The preparation splinters and the edges of breaking run along the membrane or between two half membranes.
Water is subsequently removed via freeze drying. It sublimates, i.e. it transforms out of its solid phase directly into the gas phase. This process etches the surface of the preparation. It is then coated and the metal coat is taken off and viewed in the microscope.
During the late 1970s, J. E. HEUSER (Washington University, St.
Louis) developed the quick-freeze, deep-etch preparation of samples
that avoids the crystallization of water. The (very small) specimens
are frozen quickly in liquid nitrogen transferring the water into a
glass-like state. This leads to a much better preservation of the
sample. The method is suitable for the depiction of large molecules
and molecular complexes (picture to the left). Here, too, an imprint
is gained by coating. Contrary to negative staining a
three-dimensional image of the specimen can be formed.
|
|