Depending on how many monomer subunits are present in a sugar, it belongs to the monosaccharides, the disaccharides, the oligosaccharides or the polysaccharides. Sugars contain usually one or more asymmetric C-atom, i.e. carbon atoms that carry four different substitutes. Such atoms are optically active: they shift the plane of polarized light. Sugars with more than one asymmetric carbon atom have 2n stereo-isomers (n is the number of asymmetric C-atoms).
 Sugars belong to the carbohydrates with the collective formula
Sugars belong to the carbohydrates with the collective formula
Cn(H2nOn) = (C +
H2O)n.
They consist thus, in a strictly formal sense, of carbon and water. It is distinguished between monosaccharides, oligosaccharides and polysaccharides, where several or many monosaccharide subunits are connected forming long linear or branched chains.
Simple sugars are a product of the oxidation of multivalent alcohols that contain either an aldehyde or a ketogroup. It is therefore distinguished between aldoses (aldehyde sugars) and ketoses (ketosugars).
Depending on the number of C-atoms, it is spoken of bioses, trioses, tetroses, pentoses, hexoses and so on. The naturally occurring monosaccharides have a hydoxyl-group at nearly all of their C-atoms with the exception of the C-atom that is linked to the aldehyde or ketogroup. They are hence polyhydroxyaldehydes or polyhydroxyketones.
With n = 2, glycolaldehyde is the simplest aldose. The next higher aldose is glyceraldehyde that can exist in a D- and a L-form. The first member of the ketosugars is dihydroxyacetone.
A C-atom that is linked to four different groups as glycerol aldehyde is, is called asymmetric. Such asymmetrically substituted carbon atoms are optically active, i.e. they shift the plane of polarized light. The angle of the shift is specific for the substance. D-glyceraldehyde shifts the plane to the right (+), L-glyceraldehyde (-) to the left.
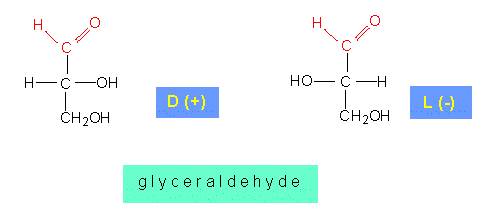
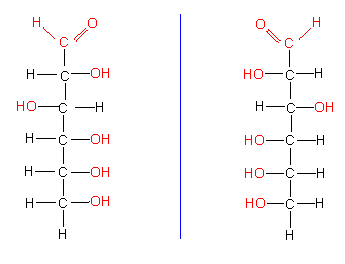
The more complex sugars with more than three carbon atoms that occur naturally do mostly belong to the D-sugars; exceptions are L-fucose, L-rhamnose, L-sorbose, etc.. This does not mean that they do all shift the plane of polarized light to the right, since sugars with more than three carbon atoms have several asymmetric C-atoms. The number of their stereo-isomers is 2n (n is the number of asymmetric C-atoms). Sugars with six C-atoms harbour accordingly four asymmetric carbon atoms. As a consequence, they have 24 different stereo-isomers with differing chemical properties. The classification of aldoses to D- or L-forms refers always to glyceraldehyde.
Carbohydrates can be depicted in several ways. One way is the linear form that has been used up till now,
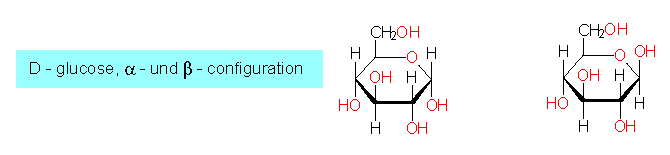
another is the ring structure that will be explained with D-glucose. The ring is a pyranose ring, i.e. it contains oxygen and it exists in two forms: alpha and beta. Both can turn reversibly into the respective other state. The C-atoms are numbered to characterize them (C1...C6). alpha-D-glucose differs thus from beta-D-glucose in the position of the OH-group at C1; alpha is below the plane of the ring, beta above.
In contrast to the ring structures of the aromatic carbohydrates or the heterocyclic rings, the pyranose ring is not plane, so that is should be written as HAWORTH's projection formulas:
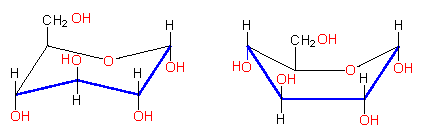
Both conformations (chair and boat) exist, though the chair form is thermodynamically more stable .
Some other monosaccharides and phosphorylated sugars:
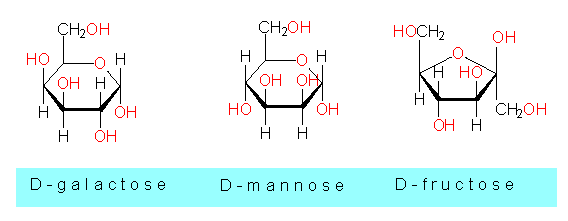
D-fructose, fructose-1,6-diphosphate,
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de