Since the second part of the previous century, dyes are used to detect certain classes of compounds in microscopy. During the thirties of this century, fluorescent dyes that could be used in much lower concentrations as 'vital dyes' became popular.
Colour molecules are usually small and their disadvantage is therefore a rather poor specificity. There exist, for example, protein-specific dyes but hardly any that are specific for a certain enzyme. A selective binding could be shown to exist for some toxins (fungi toxins, for example). Phalloidin (toxin of the amanita) binds to actin. By coupling a fluorescent dye to phalloidin, actin can thus be localized within the cell .
The different macromolecules and especially the proteins are far more specific:
Lectins. We got to know lectins in the section before last. Each lectin can be coupled to a fluorescent dye like fluoroisothiocyanat (FITC: green fluorescence) or rhodamine (red fluorescence). Such preparations are used in a big way in medicinal research and are on the market at rather low prices. They are well-suited (as we will often see in pictures presented in Botany online) as probes for the localization of glycoconjugates at cell surfaces, membrane surfaces, at compartments and others.
Macromolecules cannot easily penetrate cells. They are therefore primarily markers for extracellular surface receptors. By treatment with cellulase, for example, the cell wall of plants can be degraded, the result are protoplasts without cell walls. Depending on their origin, they bind ConA or RCA indicating that glucosyl-or mannosyl residues resp. galactosyl-residues are present at their surfaces. Protoplast preparations contain often a whole range of different cell fragments, among others also free vacuoles. The vacuolar membrane, the tonoplast, binds to no known lectin. This alone shows that it is not organized like the plasma membrane.
Fluorescence tagged lectins are especially well-suited for the mapping of lectin receptors in or at cell walls, for the proof of the cell's polarity, to detect different states of cell activity or certain states of the cell cycle; they are suited for the solubility properties of lectin receptors. WGA is an indicator for fungi mycelin in infected plant tissue.
For electron microscopic studies, the lectins have to be tagged with electron-dense markers like ferritin or colloidal gold. These complexes enable the detection of different distribution at the membrane's inner and outer surface. Lectins have also some disadvantages:
They bind to so-called glycoconjugates. It can therefore not be said without further analysis whether the receptor is an oligo- or a polysaccharide, a glycoprotein or a glycolipid.
Lectins detect only a rather small range of sugars. There exist no lectins that detect, for example, arabinose or xylose residues.
 Antibodies. Antibodies are a rather homogeneous
group of proteins that occur in the serum of vertebrates protecting
the animal from foreign influences like infections with bacteria or
viruses or from tumour cells. Plants have no antibodies. But since we
need them for the study of plant cells, will we outline them briefly:
Antibodies. Antibodies are a rather homogeneous
group of proteins that occur in the serum of vertebrates protecting
the animal from foreign influences like infections with bacteria or
viruses or from tumour cells. Plants have no antibodies. But since we
need them for the study of plant cells, will we outline them briefly:
Antibody production can be induced (immunization), i.e. a signal is needed that stimulates the animal organism to generate antibodies. Such a signal has to be of a macromolecular nature. It may be part of a cell surface. A component that induces antibody production is called antigen. An antigen is usually bigger than the respective binding site of the antibody. The antibody-binding domain of the antigen is called an antigen determinant. At an immunization, as many different antibodies are produced as antigen determinants are present. An antibody population is thus always heterogeneous or, as it is called, polyclonal.
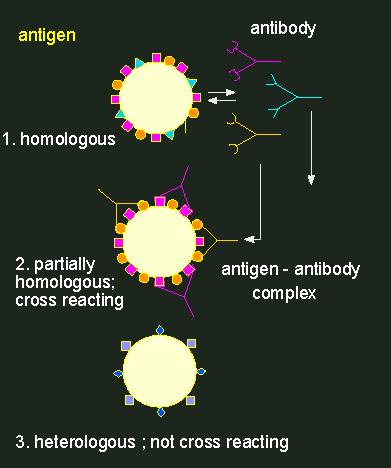
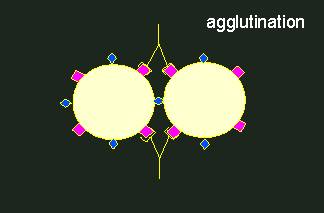
Antigen-antibody reaction.Antibodies (depicted as Y-shaped structures) form a heterogeneous population of molecules with different specificities. A cross-reaction of an antibody population (an anti-serum) with a foreign antigen (in the middle) occurs only, if the homologous and the foreign antigen are at least partially equipped with the same determinants. Every antibody has two identical binding sites for antigen determinants.
Normally, rabbits are used for the production of antibodies with a certain specificity. Two to three weeks after immunization, some of their blood can be extracted. After centrifuging the blood cells down, an antiserum is gained that contains the specific antibodies. They can now be used for the qualitative or quantitative detection of the used antigen or for the detection of substances that are similar to the antigen. In such cases it is spoken of serological cross-reactions or serological relationships. A more or less clear serological relationship is usually found in homologous proteins (enzymes, storage proteins, cytochrome C, etc.) that were gained from more or less closely related animal or plant species. The degree of serological relationship is normally related to the degree of different amino acids in the proteins.
A number of partly rather sensitive serological tests, like for example the radio immuno assay (RIA) exist.
If an antibody against a rather small molecule like a phytohormone is needed, the antigen has at first to be coupled to a larger molecule. It does thus gain the property of an antigen determinant and among the numerous antibodies produced will also be some that are directed against the phytohormone. They can easily be separated from the other antibodies that do all react with the coupled larger molecule by precipitation. The remaining antibodies will all be directed against the hormone.
To localize antigens in cells, the method that has already been described for lectins is chosen. But it is not common to tag an antibody directly with a fluorescent dye, since it is much more comfortable to produce antibodies against rabbit antibodies in another animal (for example in goats), to gain these in larger amounts and to tag them with the fluorescent dye. This is termed indirect fluorescence. Why this detour?
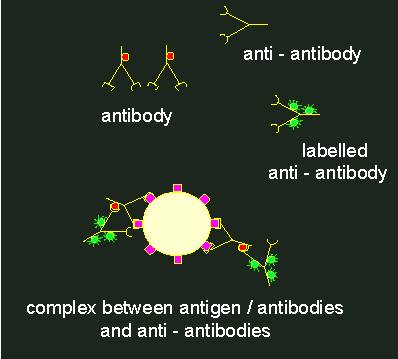
Indirect immunofluorescence.1. Specific antibody against the primary antigen. The antibodies themselves have antigen determinants (indicated by red circles). Normally, antibodies of this kind are generated in rabbits. 2. Anti-antibody (generated, for example, by the immunization of a goat with rabbit antibodies). These anti-antibodies (goat against rabbit) bind to the antigen determinants of the rabbit antibodies. If they are labelled with a fluorescent tag (green) (3), a fluorescent complex at the primary antigen is the result (4).
For electron microscopic methods again, antibodies are tagged with electron-dense materials.
However broad the range of uses in medicine, in plant research antibodies are still used only rarely. One of the main reasons is the cell wall that cannot be penetrated by any antibody, the other reason is the lack of well-suited antigens. But still, several good results do exist. Phytochrome, a light receptor that is among the most-important control units in the plant could be localized in certain cells. It could also be shown that it is missing in others.
To mark cell contents, the cells have either to be cut into slices or protoplasts with partially permeable membranes have to be generated. Among the other antigens localized by immunofluorescence are some enzymes like phosphoenol pyruvatcarboxylase, alpha-amylase and several storage proteins and cytosceleton elements.
Since their detection by C. MILSTEIN and G. KÖHLER (Medicinal Research Council, Laboratory of Molecular Biology, Cambridge and Basel Institute of Immunology) in 1975, monoclonal antibodies are regarded as the non plus ultra. Monoclonal antibodies are homogeneous antibody populations that are produced in cell cultures and that display a very narrow specificity (against only one determinant). About their production:
Antibodies are generated in small lymphocytes. One of the tissues with most lymphocytes is the spleen. Several days after immunizing, for example, a mouse is its spleen removed and a cell suspension is generated. These cells are then fused with myeloma cells of mice (one of many tumour lines). The fusion produces hybrid cells (hybridoms). Myeloma cells are characterized by unlimited growth. This property is also kept by the hybridoma cells. But they have the additional property of antibody production and secretion. In an intermediate step is a wanted cell line isolated and cultivated.
The method has mainly one disadvantage: the antibodies are highly specific but the number of binding sites (antigens) of the cells is drastically reduced. Consequently, only very few antibody molecules are bound, often is the antigen-antibody reaction at the limit of provability. But here, too, exist ways to success: in indirect fluorescence, TV cameras with electronic image intensification are used.
Detection of Enzyme Activities. Can enzymes or substrates in the cell also be detected without antibodies?
Different methods exist. Most common is autoradiography. For this, a radioactively tagged substrate or, even better, a substrate analogue that is bound by the enzyme but not converted is needed. After washing off the material that has not been bound, an autoradiogram can be produced with the help of a special film that shows the position of the enzyme. The method is especially well established with membrane bound enzymes.
Another possibility is the use of a substrate that is processed and renders an insoluble, stained product. In electron microscopy again, electron dense markers are used.
|
|