In 1892, the Russian D. IWANOWSKI discovered horizontal transmission. He succeeded in infecting plants with the tobacco mosaic disease by applying a bacteria-free sap gained from infected tobacco leaves. This discovery was confirmed some six years later by the Dutchman M. W. BEIJERINCK. He filtered an extract of infected plants through a porcelain strainer not viable for bacteria, and found that the sap thus obtained displayed no loss of its infecting ability. The virus itself was not isolated till 1935 and not crystallized before 1937. In 1937, F. C. BAWDEN and N. W. PIRIE (Rothamsted Experimental Station, England) detected that the preparations contained phosphate, an inherent part of the RNA molecule.

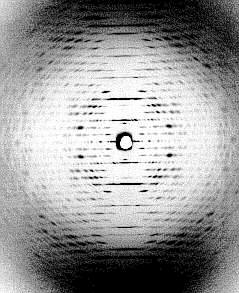
At the left: crystalline arrangement of TMV particles in the mesophyll cells of tobacco, side view (J. H. M. WILLISON, Halifax, 1976). At the right: diffraction image of a highly concentrated TMV-solution. All virus particles are arranged in parallel (K. C. HOLMES, Heidelberg, 1970).
A number of TMV wild types differing, for example, in their host range or in the primary structure of their coat proteins exist. The classic strain that at the same time is the one that proliferates best on tobacco plants is called vulgare. A strain from tomatoes that proliferates equally well on tobacco plants is called dahlemense (G. MELCHERS, then at the Kaiser-Wilhelm Institut für Biologie at Berlin-Dahlem). A third strain gained from plantain is known as Holmes ribgrass (HRG; F. O. HOLMES, 1934).
The single strains differ visibly in the symptoms they cause in tobacco plants. The amino acid sequences of the coat proteins of four different wild strains show that a certain sequence remains always unchanged. This sequence is assumed to be in direct contact with the RNA. The analysis of the coats’ tertiary structures confirmed this assumption.
A number of mutants was generated by the use of mutagenes. Some of these mutants differ from the 158 amino acid-long coat sequence of the wild type only in one or two amino acids. The existence of a large number of just slightly differing proteins answered a number of further questions.
Is, for example, the replacement of just one of the 158 amino acids sufficient to detect the mutation serologically?
Do the amino acid replacements happen statistically, i.e. changes every amino acid independent of its place in the polypeptide chain with the same frequency or are certain sequences preferred?
Occur changes in the stability of the polypeptide after the replacement (exchange) of single amino acids? and
Do changes in the virus coat change the symptoms of the tobacco plant?
On 1. All mutants cross react with a serum against the wild type. The strength of the reaction, nevertheless, does differ. Some mutants cannot be distinguished from the wild type by their serum reaction, while others display clear differences. An exchange of Pro 20 against Leu can serologically not be detected while the same exchange at position 156 shows a marked difference. These experiments proved that amino acid exchanges do only have a serological effect if the exchanged amino acid is positioned directly at the particle’s surface or if the change alters the conformation of the particle surface indirectly. Röntgen structure analysis (X-ray analysis) of the tertiary structure confirmed this result originally made in 1965.
On 2. The changes of the polypeptide caused by mutagenes do not occur statistically, but concentrate on certain sequences. It looks as if exchanges in other parts of the polypeptide would cause so much damage to the tertiary structure of the polypeptide that no stable quaternary structure (no intact three-dimensional coat) can be formed. As a consequence, the viral RNA is not sufficiently protected against plant RNAses and the mutant viruses will die. Under special experimental conditions, mutants with a defect or irregularly assembled coat protein develop.
.....................................10 .....................................20 SER TYR SER ILE THR THR PRO SER GLN PHE VAL PHE LEU SER SER ALA TRP ALA ASP PRO ............................................................................LEU .....................................30......................................40 ILE GLU LEU ILE ASN LEU CYS THR ASN ALA LEU GLY ASN GLN PHE GLN THR GLN GLN ALA VAL ............SER ............................SER............................ .....................................50 .....................................60 ARG THR VAL VAL GLN ARG GLN PHE SER GLN VAL TRP LYS PRO SER PRO GLN VAL THR VAL ....................GLY.................................................ILE.... .....................................70 .....................................80 ARG PHE PRO ASP SER ASP PHE LYS VAL TYR ARG TYR ASN ALA VAL LEU ASP PRO LEU VAL ........SER.... GLY GLY........................................................ .....................................90.....................................100 THR ALA LEU LEU GLY ALA PHE ASP THR ARG ASN ARG ILE ILE GLU VAL GLU ASN GLN ALA ALA.............................................................GLY.....ARG.... ....................................110 ....................................120 ASN PRO THR THR ALA GLU THR LEU ASP ALA THR ARG ARG VAL ASP ASP ALA THR VAL ALA ........................MET.................................................... ................................... 130 ....................................140 ILE ARG SER ALA ILE ASN ASN LEU ILE VAL GLU LEU ILE ARG GLY THR GLY SER TYR ASN ....................SER ........THR.........................ILE ....PHE.....SER ....................VAL........................................................ ....................................150.............................158 ARG SER SER PHE GLU SER SER SER GLY LEU VAL TRP THR SER GLY PRO ALA THR ............................PHE.............................LEU.....---
The amino acid sequence of the tobacco mosaic virus (vulgare; upper line).The lower line shows only possible amino acid exchanges (in blue) . Such exchanges can be found in a number of analyzed mutants. An antiserum against the wild type detects the red amino acid exchanges. See the text for more details or click here. More about tertiary and quaternary structures can be found here.
On 3. The wild type protein tolerates increased temperatures during assembly into virus particles. The virus is therefore able to multiply at higher temperatures (around 30° C). Many of the mutant coat proteins are temperature-sensitive, i.e. no regular quaternary structure develops at risen temperature. Such mutants multiply only at normal temperatures (up to 20° C): they are conditionally lethal (H. JOCKUSCH, Tübingen, 1964).
On 4. Several interesting observations have been made. The coat protein of almost all strong "yellow strains" is charged more positively than that of the respective wild type protein. The following amino acid replacements cause the development of yellow strains: Asp > Ala, Asp > Asn, Asn > Lys. Whether these exchanges have a direct impact on the processes involved in symptom development, or whether they are merely concomitant is not known yet.
In order to start replicating within a cell, the RNA has to be released from its protein coat. The + strand of the RNA serves as a matrix for the production of a complementary strand. The new complementary – strand is the template for the production of numerous new + strands (T. NILLSSON-TILLGREN, Institute for Genetics, Copenhagen, 1970, 1974). Replication takes place within the cytoplasm. The replicase’s affinity for the – strand is several orders higher than its affinity for the + strand resulting in many + strands but only few – strands.
Parts of the + strand are used as mRNA. . The gene for the coat protein does not belong to them. The – strand does not only serve as a template for the production of + strands, but also as a blueprint for a short messenger of the coat protein. The Turnip-Yellow-Mosaic Virus (TYMV) takes a similar way, while the complete information of the RNA-strand is read en bloc in the case of the tomato black-ring-virus (TBRV) and the cowpea virus (CPV). Subsequently, the transcription product is cut proteolytically into smaller polypeptides.
Meanwhile, the – sometimes only partial – nucleotide sequences of a number of virus species are known. Parts of the RNA molecules are able to fold into a secondary structure resembling a tRNA. RNA of this type has the ability to bind certain amino acids, which amino acids exactly depends on the virus species. It remains unknown whether this phenomenon is linked to the symptoms displayed by the infected plant or to the replication of the viruses.
Interference:
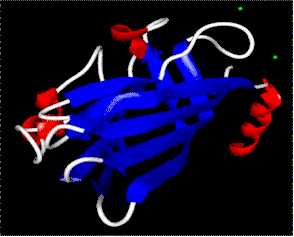
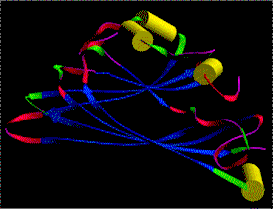
The densely packed nucleic acid of many spherical viruses is enclosed by a protein coat also called capsid. The capsid is a polyhedral, i.e. it has many sides. The exact number is specific for the respective species. The aggregated polypeptides form morphological units often composed of several identical polypeptide chains. The crystal structures of a number of different RNA-containing plant viruses are known, among them are:
the tomato bushy stunt virus (TBSV),
the southern bean mosaic-virus (SBMV),
the satellite of the tobacco necrosis virus (STNV),
and the turnip crinkle-virus (TCV).
All of them have an icosaedrical structure. They consist of 60 (= 5 x 12) identical polypeptide copies of with the same building pattern assembled to form a hollow sphere. Structural models of spherical viruses can be found at:
Simple examples of this type of architecture are the SBMV and the TBSV. The polypeptide chain of the TBSV contains 386 amino acids, the tertiary structure with its high degree of beta-sheets looks somewhat strange. Four domains can be distinguished. The innermost part (R) is always charged positively and extends into the sphere. A bridge links it to the domain S that forms the original coat (S for shell). The fourth domain protrudes from the virus (P for projection) and gives the virion its spiky look. The basic assembly pattern is the same as that of the SBMV, although its proteins structure is far more complex. Here, too, the structure consists of three subunits, 60 of which form a capsid. The capsid subunits of the bean pod virus contain two polypeptide chains each.
A number of RNA plant viruses contain more than just one molecule of RNA per virion ( a common phenomenon in animal viruses, the influenza virus being a typical example). In others, the genome is distributed among several virus particles.
Case 1: The cucumber mosaic virus (CMV) is an example of the first type. It contains five molecules of RNA, four of which are required for the replication of the virus, the fifth is likely to have a helper function and can be classified as satellite-RNA (CARNA 5). It carries genes that can be expressed. Infected tobacco plants produce large amounts of this satellite-RNA (CARNA 5). An in vitro-system helped to identify two polypeptides with an effect on the seriousness of the plant’s infection. The degree of the effect these two polypeptides have depends on the host plant. In contrast, CARNA 5 leads to the lethal tomato-necrosis-disease in CMV-infected tomato plants. An epidemic in France lead to the isolation of large amounts of CARNA 5 (J. M. KAPER, US Department of Agriculture, Beltsville, Maryland, 1977).
Case 2: Incomplete viruses, satellite viruses: The multiplication of viruses leads often to incomplete particles. TMV preparations show usually an abundant amount of particles of variable length. Neither of them is infectious. They contain often cellular RNA instead of a complete or partial TMV-RNA. This frequently high proportion of non-infectious particles makes it very difficult to determine the plating efficiency of the virus.
The genome of the alfalfa mosaic virus (AMV) consists of three molecules of RNA contained in three capsids (the B, M, and Tb-particle). All three particles together are infectious, the RNA isolated from them alone is not infectious, but it regains its infectivity if the coat protein is added. It seems to be necessary for the initiation of the infection process.
In satellite viruses, the infectiousness depends on the presence of a helper virus. The tobacco-necrosis virus (TNV) and its satellite (STNV) are a typical example. The multiplication of the TNV is not dependent on the presence of STNV. TNV alone produces large lesions in tobacco plants. These lesions are small if STMV is present. It seems that STMV inhibits the distribution of TNV (B. KASSANIS, 1962).
|
|