The microfilament system, also called the actin-myosin system, was originally discovered as the principle underlying muscular contraction. At the beginning of the sixties evaluated H. E. HUXLEY (Medical Research Council, Laboratory of Molecular Biology, Cambridge/ England) electron microscopic pictures of muscle cells. He was thus able to shed light upon the molecular structure of the muscle cell and he could, too, explain muscle contraction as a telescope-like gliding past each other of parallel filaments. As soon as actin and myosin are in contact, starts myosin to perform its ATPase function.
Later on was actin proven to be one of the most common proteins of all animal cells. Actin can be detected via three different ways:
Binding of myosin. A myosin molecule consists of two parts, a head out of a folded polypeptide chain with a specific tertiary structure and a tail where the polypeptide chain is a long, uninterrupted alpha-helix. Treatment with trypsin separates the heads and allows their isolation. They do not loose their ability to be taken up by actin filaments. In the electron microscope (negative staining) can the actin-myosin complex be made out by its characteristic, arrowhead-shaped appearance. Since the arrowheads taken up by an actin polymer do all display the same orientation does it seem likely that the actin molecule has a polarity. The method's disadvantage is its requirement of more or less purified actin filaments.
Indirect immunofluorescence. The technique was developed by E. LAZARIDES and K. WEBER (at that time at Harvard University, Cambridge/Mass.) in 1974. It became one of the most successful methods of modern cell biology. Actin is a relatively well preserved protein, i.e. an antibody population directed against animal actin reacts with the actin of nearly all animals including moulds. Compared with actin detection via myosin binding has this technique the advantage of depicting the complete microfilament system of a cell, as well as capturing changes in the course of the cell cycle or in the organism's development. The method requires that the antibodies are inserted into the cells. In the standard procedure are the cells at first fixed with glutaraldehyde and afterwards is the membrane dissolved or at least made permeable for molecules the size of antibodies. Another possibility is the microinjection of living cells with labelled antibodies. The use in plant cells is limited due to the cell wall. Protoplasts are a possible way out of the dilemma.
Labelling of microfilaments with fluorescence-tagged phalloidine, the destroying angel's (Amanita phalloides) poison. Phalloidine has a high affinity for the F-actin of liver and muscle cells, with which it forms stable complexes. The fact that it binds plant actin with the same high affinity offers a way out of the limited availability of plant antibodies without a loss in specificity.
As has been mentioned before is the F-actin of animal cells associated with a number of different proteins: myosin, troponin (A, B, C), tropomyosin, alpha-actinin and others. Little information about their occurrence in plant cells exists. Myosin, or a myosin-like compound seems to have been found those plants investigated (Nitella, Lycopersicon).
Several inhibitors of microfilaments exist. One of the best-known is cytochalasine B, a mycotoxin, that inhibits the polymerization of actin. In cell biology is it often used to distinguish between the microfilament and microtubuli systems.
It seems certain that microfilaments participate in the protoplast currents of the stoneworts Chara and Nitella's internodal cells, in the chloroplast movements of Vallisneria and maybe in the pollen tube growth and the nuclear migration of Acetabularia, too. Especially the last example shows that microtubuli are involved in that movement, too. Both systems co-operate tightly (H. U. KOOP and O. KIERMEYER, Universität Salzburg, 1980).
In the middle of the eighties identified M. V. PARTHASARATHY and his collaborators (Cornell University, Ithaca, N. Y., 1985) microfilaments of a range of plant types with the help of phalloidine tagging. They could prove that microfilaments are very common in plants, too, and that they develop clearly structured cytosceletons in the cells. They form indeed the basic scaffolding of the plasma strands, participate in plasmatic movements, are in direct contact with plastids and occur abundantly in pollen tubes. The nucleus is surrounded by a closed network of microfilaments, the nuclear basket.
In moulds and their coenocytes partakes actin in the amoeboid movements. It causes the intense, pulsating movement of the plasma. In the plant kingdom are amoeboid movements rare, pollen tube growth could maybe compared to them. If they are, would it be remarkable because it would mean that actin takes part in the fertilization process of higher plants (in lower plants participate flagellate spermatozoons and their movements are caused by microtubuli).
Chara and Nitella: Their giant, several centimetres long and roughly one millimetre thick internodal cells have ever since they have been discovered been popular with movement physiologists since their plasma (except the plastids) is constantly rotating. The plastids are arranged in an immobile, cortical pattern.
During the thirties detected K. ARENS (University of Rio de Janeiro) a 'physiological multipolarity' of the cells. He observed that calcium ions which may form chalk deposits can be secreted along the cell wall at regular intervals. From what is known through the study of animal, especially muscle cells are calcium ions essential regulators of the actin-myosin interaction The question whether they have the same regulatory effect in Nitella or Chara is still unsettled, but the following experiment (see diagram) shows that the 'physiological multipolarity' indeed is correlated with the plasma current.
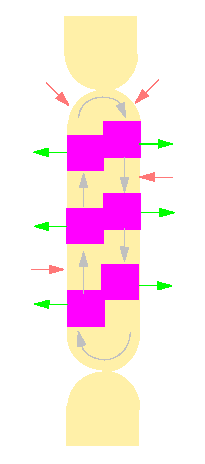
Scheme of the physiological multipolarity of an internodal cell of Nitella. The arrows within the cell give the plasma current's direction. Magenta areas are zones where calcium ions are secreted at the surface of the cell. In the light areas are calcium ions taken up (according to K. ARENS and R. JAROSCH, 1939).
In 1958 detected R. JAROSCH (Mikrobiologische Station der Stadt Linz, Austria) protein fibrils in the pressure sap of Chara cells (visible in a dark field microscope). He understood that they form rotating rings and are often studded with other plasma particles. JAROSCH took them for the impulse carriers of plasma movements, as he called them, since he could show that the movement spread impulse-like along the ring. Years later (in 1972) detected E. KAMITSUBO (Osaka University) that such rings develop within cells after centrifugation, too. They were also characterized by an autonomous, rotating movement. The observations point at the possibility that the saltatory movements of cell organelles (mitochondria, microbodies and others) could be due to their binding to rotating fibrillar systems.
The improvement of microscopic techniques allows to determine the position of protein fibrils within cells of Chara or Nitella. The results of studies with phase contrast, interference contrast or scanning electron microscopy are especially impressive.
The pictures show that the fibrils are arranged in rows of three to four parallel filaments at the border between stationary ectoplasm (with fibrils) and mobile endoplasm. In the middle of the seventies was it finally proven that they do indeed contain actin (R. WILLIAMSON, 1974, B. A. PALEVITZ, J. F. ASH, P. K. HEPLER, 1974, B. A. PALEVITZ and P. K. HEPLER, 1975) and that all parallel microfilaments are oriented alike. The result was supplemented by a labelling with monoclonal antibodies. Corresponding to our knowledge of muscle cells is the velocity of the endoplasm current dependent on ATP.
The latest method of light microscopy is confocal laser scan microscopy where the objects are captured layer by layer and are evaluated with the help of specific software. The depiction of fluorescence-tagged, mobile objects like the elements of the endoplasmatic reticulum, other membrane systems (the Golgi-apparatus, for example) or the cytosceleton are quite impressive. They show that bundles of microfibrills are tightly connected with the ER and that the Golgi-apparatus, for example, moves along the filaments as if on rails. An article by Cris HAWES (Oxford Brookes University) with video sequences about this topic can be found at:
Epidermal cell cortical actin network (red): By reducing the green signal and removing ER fluorescence the Golgi can be seen aligned on actin cables. Rhodamine phalloidin staining revealed a cortical network of actin filaments which were overlaid by the cortical ER network. By reducing the contrast of the ER in the green image of these double labelled preparations it could be seen that the Golgi bodies are aligned on actin cables (= associated with this actin networ).© C. HAWES, A. BETTERIDGE, P. BOEVINK, S. SANTA CRUZ & K. OPARKA
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de