Pattern formation within a segment: dynamic regulation of a neighbourhood of structures
Mutual activation of cell states that locally exclude each other
Segmentation: the repetitive pattern of (at least) three cell states
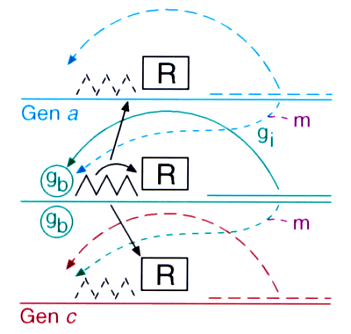
Fig. 9: Space-dependent gene activation by a morphogenetic signal. Predicted scheme: alternative genes are supposed to have an autocatalytic feedback on their own activity but compete with each other, for instance, by a common repressor R. Both the autoregulation of genes involved in differentiation and the stepwise promotion has been meanwhile confirmed.
The generation of signals by the exchange of molecules via diffusion works only in small fields. In larger fields the time required to exchange information by randomly moving molecules would be much too long. Indeed, the sizes of embryonic fields in which developmental decisions take place are always small, smaller 1 mm and less than 100 cells across (Wolpert, 1969). Therefore, the signals generated at small scales have to be translated into more permanent states of differentiation that can be maintained upon further growth. The obvious means is a stable concentration- (and thus space-) dependent activation of genes. The choice of a particular pathway under the influence of a morphogenetic signal requires the activation of particular genes and the suppression of alternative genes. This situation has formal similarities with the formation of a pattern: pattern formation requires activation at a particular position and the inhibition in the remaining part. The selection of a particular pathway requires the activation of a particular gene and the suppression of the alternative genes. Based on this similarity, I have proposed that gene activation requires a direct or indirect feedback of genes on their own activation and their mutual competition such that only one of the alternative genes can remain active in a particular cell. (Fig. 9, 10); Meinhardt, (1978). Meanwhile many such autoregulatory genes have been found. The genes deformed (Regulski et al., 1991), hunchback (Simpson-Brose et al., 1994) or twist (Leptin, 1991) are examples.
Such a dynamic regulation of gene activation has many properties that are essential for the developing organism. Firstly, such a system has a threshold. The activation of a gene becomes an all-or-nothing event. If a threshold is surpassed, due to the autoregulation, the response of the cell becomes independent of the exact level of the morphogen concentration. Therefore, small deviation from a desired signalling strength are not propagated into the hierarchically next level of gene activation. Secondly, the cells obtain a long-term memory in respect to the signals they have seen. The signal is required only for the initiation of gene activity. Due to the feedback, the activity is self-maintaining.
For the translation of a graded concentration profile of a signal substance into the position-dependent activation of genes I have proposed that the cells do not measure a particular morphogen concentration all at ones. Instead, starting from a default gene activity, other genes become activated in a step-wise manner. Each further step requires a higher morphogen concentration. This process comes to rest if the actual gene activation corresponds to the local morphogen concentration (Fig. 10) (Meinhardt, 1978).
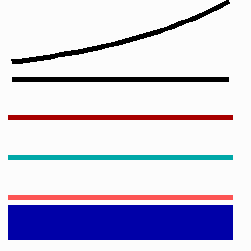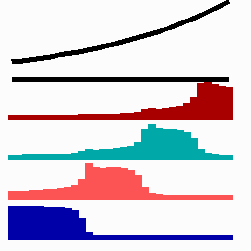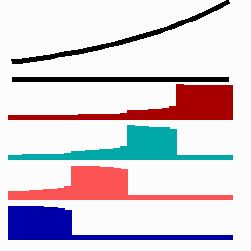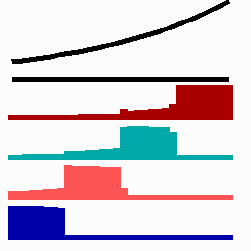
Fig. 10: Gene activation by a morphogen gradient (black, top). Starting with a default gene (blue), a "promotion" from one gene to the next under control of a morphogenetic gradient leads to sharply confined regions in which particular genes are active.
Based on this mode of gene regulation, regulatory features have been predicted that have been recently observed. For instance, shifting a cell from low to a high concentration is expected to cause a subsequent "promotion", i.e., an adaptation to the new morphogen concentration should occur. Gurdon et al. (1995) found that a low concentration of Activin (a member of a family of growth factors) causes in cells from the blastula stage of Xenopus (an amphibian) an activation of the Xbra gene while for the gene Xgsc a high concentration is required. Applying Activin first in low and later in high concentrations leads to a reprogramming of cells from Xbra to Xgsc. Gurdon et al called this a ratchet-like switching, in full agreement with the mechanism proposed (Fig. 11a).
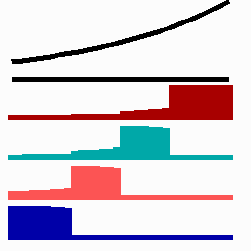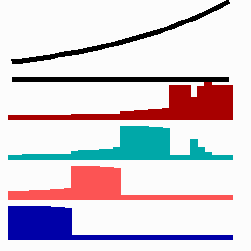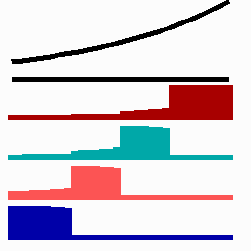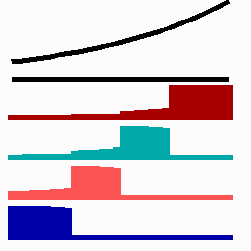
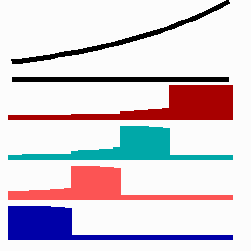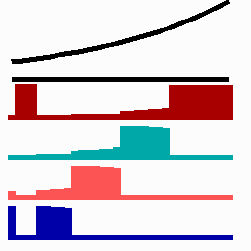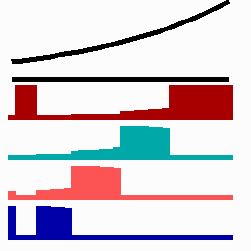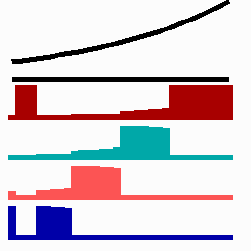
To the left - Fig. 11a: Re-specification of a cell after transplantation into a region of higher morphogen concentration. At the new position, cells become promoted and obtain a specification according their new environment
To the right - Fig. 11b: Stability of a the gene activity upon transplantation of a cell from a region of high to region of low morphogen concentration.
Due to the autocatalysis of the gene activation and the unidirectional promotion, a once obtained gene activation is essentially irreversible. A reduction of the signalling molecules is without effect since the morphogen is not required for the maintenance of the gene activity (Fig. 11b). This mode of stepwise promotion has a very essential function during development. As mentioned, the range of the signalling molecules is usually small. The initial activation of genes takes place in small fields that grow afterwards. During growth, the distance between a cell and the morphogen source necessarily increases and thus the morphogen concentration decreases. Due to the irreversible character of the stepwise promotion the cells maintain their once obtained state of determination even if they escape from the influence of a morphogen source. This stability is possible without that the determination is absolutely fixed. If, for instance, during regeneration, a new morphogen source is generated, the cells can still be "distally transformed". In the regeneration of a cockroach leg, this process plays a decisive role. The step-wise and irreversible promotion enables on the one hand the required stability to cope with the fading influence of the morphogen during growth and provides, on the other, still some (unidirectional) flexibility if regulation is required. This avoids the necessity of a narrow time window to which the measurement is confined.
Meanwhile several systems with this behaviour have been described. The specification of the hindbrain follow that scheme (Gould, 1998, Grapin-Botton et al., 1998). Retinoic acid mimics the natural morphogen. A transplantation to a posterior position, i.e., towards the presumptive morphogen source, leads to an adaptation to the new position, while a the pattern of gene activation is maintained after a transplantation to a more anterior position, i.e. away from the source.
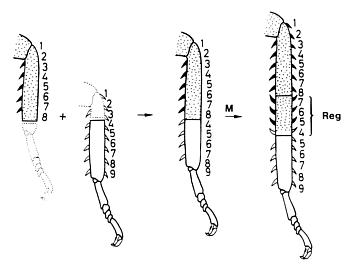
Fig. 12: Experimental evidence that a correct neighbourhood is involved: A discontinuity resulting from grafting two parts of a cockroach leg together is smoothed out by intercalation of the corresponding structures at the zone of juxtaposition (after Bohn, 1971).
A widespread pattern in biology is segmentation, the reiteration of similar structures. The body segments of insects or the segments of insect legs are examples. Segments have an internal pattern, frequently visible by overt structures such as bristles or spines. The pattern within a segment has characteristic landmarks. Information about how the pattern within a segment is regulated has been obtained from surgical interference in hemimetabolous insects. These are insects that have from beginning an appearance similar to the adults but no larval stages. The juvenile forms proceed through several moults. Creating a discontinuity by grafting a piece of the sclerotic cuticula together with the underlying ectoderm to a different position within the segment provokes pattern regulation (Locke, 1959). After one or two moults, a new stable pattern becomes established. The result of such regulation is a restoration of the normal neighbourhood of structures: the discontinuity disappears. However, the resulting pattern can be dramatically different from the natural pattern. Fig. 12 shows an experiment of Bohn (1970) with cockroach legs that provides an example. If we denote the normal sequence of structures within a segment as 123...9, a combination of a stump 12345678 and a grafted piece 456789 leads to the structure 12345678765456789. Although the leg was already too long after the operation, even more structures became inserted by intercalary regeneration (written in bold face). After this intercalation each structure has a neighbour that would be also a neighbour in the non-perturbed situation. Obviously, it is not the natural sequence of elements but the normal neighbourhood that is regulated. This argues against mechanisms according to which a segment is organized by a gradient generated by a source at one segment border and a sink at the other since, after a manipulation as described above, a restoration of the normal monotonic sequence would be expected. As indicated by the reversed orientation of the spines (Fig. 12), the surplus structures are intercalated with a reversed polarity. Thus, the polarity of the pattern within a segment results from the sequence of elements and not from the alignment of polar cells since polarity reversal can occur without rotation of parts.
The possibility to generate a sequence of structures by gene activation under the influence of a gradient has been outlined above (Figs. 9, 10). In such a mechanism, the cells do not communicate directly with each other to obtain the correct determination. They measure the local concentration of a substance and behave accordingly. Due to this lack of communication, as a rule, a discontinuity can not be repaired, especially if at later stages the signalling gradient is no longer available. The cell just remain in the once obtained state. A gradient mechanism is therefore inconvenient to account for a dynamic regulation of a correct neighbourhood.
As shown above, self-activation of genes together with a mutual repression of alternative genes leads to stable states of determination. If two (or more) such states not only exclude each other locally but activate each other mutually over long ranges, these cell states stabilize each other in a symbiotic manner. Neighbouring cell states need each other close by while the local exclusiveness assures that these states do not merge or overlap (Meinhardt and Gierer, 1980). The most stable state is reached if a region in which a particular gene is active is bordered by regions in which the genes for the correct neighbouring structures are active. According to this model, segmentation requires the following molecular ingredients:
The theoretical prediction found meanwhile direct support (Fig. 13). In Drosophila the gene engrailed (en) is a key gene for segmentation. It has a direct autocatalytic feedback on its own activation. Further, it activates a neighbouring cell state via a diffusible molecule hedgehog. This cell state is characterized by the activation of the gene wingless (wg). The wg protein can also diffuse into neighbouring cells and stabilizes en. As expected from the theory, the en gene activity requires a functional wg gene in its neighbourhood and vice versa although both genes are transcribed in non-overlapping regions. The prediction of such a complex molecular interaction by a theory could hardly be more precise. From the theory we would expect that the wingless gene is under transcriptional control of a second (directly or indirectly) autocatalytic gene. The gene ci on which wingless-expression depends and that is repressed by en is perhaps a part of the missing feedback system (for references, see Meinhardt, 1994). A simulation under this assumption is given in Fig. 14a. It is a feature of these interactions that they can generate stripes. A stripe-like gene activation causes long borders between the regions. A particular cell type is close to the cell types that are required for stabilization. This facilitates the mutual support by the diffusible substances. A stripe-like arrangement is therefore especially stable. Many genes involved in the segmentation of Drosophila are expressed in narrow stripes.
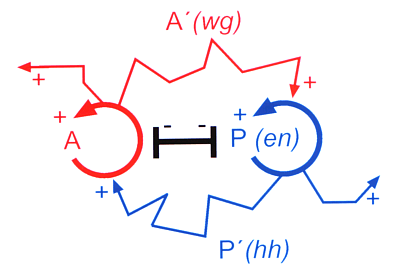
Fig. 13: Reaction scheme as required for segmentation (Meinhardt and Gierer, 1980). Predicted was that the correct activation of genes for adjacent structures is based on feedback loops (circular arrows) that locally exclude each other (black T-bar) but on long range mutually activate each other by substances that are exchanged between neighbouring cells (zigzag-arrows). This prediction has found meanwhile strong support from the experimental side. The key gene for the posterior compartment, engrailed (en) has an autoregulatory component, the long range help for the anterior compartment is based on hedgehog.The help of the anterior onto the posterior compartment (i.e., on en activation) works via wingless.

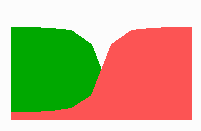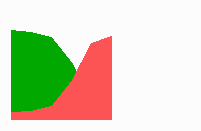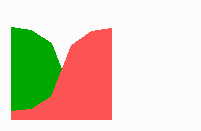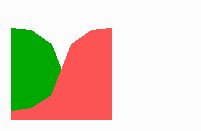
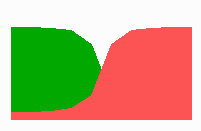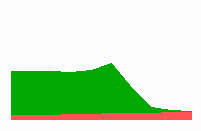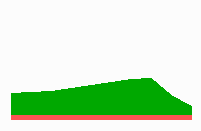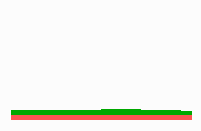
Fig. 14a: Pattern formation by a local exclusion-long range activation mechanism.
Fig. 14b: Pattern regulation after partial removal of one region.
Fig 14c: Breakdown of one feedback loop leads to a collapse also in the other since both depend on each other although they are active in different parts of the field
Fig. 14a-c shows the dynamic behaviour of two such locally competing feedback loops that activate each other on long range. Minor asymmetries are able to initiate pattern formation (Fig. 14a). Since both loops have equal rights, there is no longer an activated and a non-activated region. The system has the capability of size regulation. Partial removal of a region in which one loop is active (red in Fig. 14b) leads to pattern regulation with the expansion of the region that is to small on the expense of the larger one. This size regulation works only if elements of the autocatalytic loops can be exchanged between cells. If this is not the case, the border between two specification cannot be shifted. The progeny of a cell will maintain a once obtained specification, i.e., the obey a lineage restriction. The border between two such specifications act as compartment border. This is situation in insects since the transcription factor engrailed is restricted to the nucleus and cannot exchanged between the cells. ;
If one of the competing loops is non-functional due to a mutation, the second loop will become extinct too (Fig. 14c) although there is no longer a competitor present. But there is also no longer the obligatory help from the other cell state.
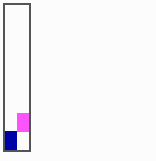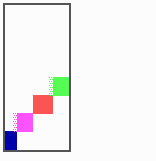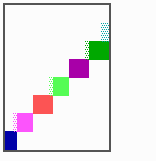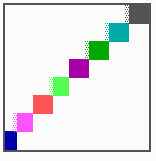
Fig. 15: Generation of a sequence of determination by mutual activation and local exclusion. Whenever a structure is large enough, the help leads to the induction of the subsequent structure (Meinhardt and Gierer, 1980)
The simplest periodic structure would consist of an alternation of two cell states, let us say, A and P. The two compartments found in Drosophila appear to support such a view. However, such a binary sequence has no polarity, in contrast to the biological observation. No signal would be available that indicates at which AP-border a segment border has to be formed; the grouping could be AP/AP/AP or ..A/PA/PA/P.. Both sequences would have opposite polarity. This problem has two possible solutions: (i) an additional pattern with a repeat length of two segments exists, consisting for instance of a pattern ..OEOE..; O and E would each cover the domain of a future segment and would eventually coincide with either an even (E) or an odd (O) numbered segment. Each O and E state would be subdivided into an A and a P region. The signal to form a border would be an O/E but not an A/P confrontation. (ii) Segmentation results from the periodic alternation of (at least) three cell states, for instance S, A and P. A segment border would be formed whenever P and S cells are juxtaposed (...P/SAP/SAP/S...). A cyclic sequence of (at least) three cell states has necessarily a polarity since each cell state has anteriorly a different neighbour than posteriorly. Although a double segment pattern exists in Drosophila, the segment borders are determined by the segment polarity genes, not by the pair rule genes (for further details in the modelling, see Meinhardt, 1986).
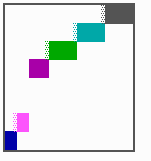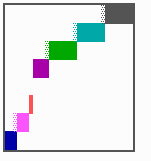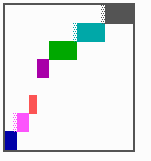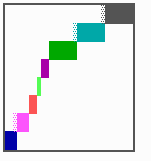
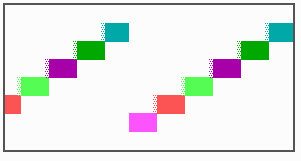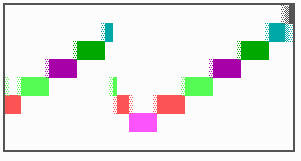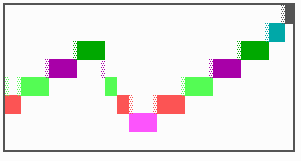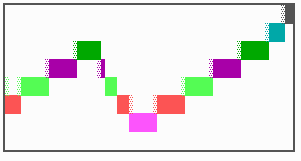
Fig. 16: (a) A gap in the sequence of structures is repaired by the induction of the missing elements (a). (b) If surplus structures are present (see Fig. 12), this is accompanied by a polarity reversal.
The systems has interesting properties if more than two feedback loops are involved. In simulation Fig. 15, the generation of a sequence of gene activations is shown that works without a global, long ranging molecule. By accretion of new cells at one border, new feedback loops are activated whenever in a sufficient number of cells the preceding loop was active. The resulting sequence of gene activation is self-regulating; missing structures become intercalated (Fig. 16a), if necessary with polarity reversal (Fig. 16b), in full agreement with the observation (Fig. 12).
The mutual long ranging activation of cell states that locally exclude each other is in its core an alternative version of the local autocatalysis - lateral inhibition scheme (Gierer, 1981). In this case, the inhibition works by a long ranging activation of a competing cell state.