In 1934 succeeded P. R. WHITE (Rockefeller Institute, Princeton, N.Y.) in cultivating of tomato root tips and meristems (Lycopersicon esculentum) under water, i.e. submerged. The medium contained inorganic salts, glucose and yeast extract. The latter was later on replaced by three of the B-vitamins (thiamine, pyridoxine and nicotinic acid).
At about the same time detected R. J. GAUTHIERET (Faculté des Sciences de Paris) that the cambium of willow (Salix) and other lignified plants turns into a not normally differentiated scar tissue also called callus. A callus is an irregularly structured mass of cells whose rates of differentiation and growth differ. Some parts stay meristematic while others sclerify. Sclerification leads finally to the dying of the respective tissue part.
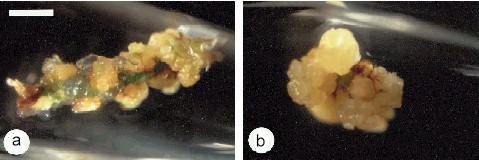
Callus Explants from Root Pieces of Cymbidium ensifolium. The bar represents 2.5 mm. C. CHANG and W. C. CHANG, 1998 - Plant Cell Reports, 17, 251-255 (1998) - © Springer-Verlag
In 1939 began P. A. C. NOBÉCOURT (Paris) the first permanent callus culture from root explants of carrot (Daucus carota). Such a culture can be kept forever by successive transplantations onto fresh nutrient agar. The transplantations occur every three to eight weeks. Callus cultures are no cell cultures, since whole tissue associations are cultivated. Though many cells keep their ability to divide, is this not true for all. One reason for this is the aneuploidy of the nuclei and the thus caused unfavourable chromosome constellations.
J. van OVERBEEK (Rijksuniversiteit Utrecht) introduced in 1941 coconut milk as a new component of nutrient media for callus cultures. Coconut milk is liquid endosperm. In nature does it stimulate the embryo to grow which it supplies at the same time with food. Results yielded from callus cultures showed that its active components stimulate the growth of foreign cells, too.
In 1954 developed F. SKOOG (University of Wisconsin, Madison) a technique for the generation and culture of wound tumour tissue from isolated shoot parts of tobacco (Nicotiana tabacum). The thus developing callus grows when supplied with yeast extract, coconut milk or old DNA preparations. Freshly prepared DNA has no effect but becomes effective after autoclaving. This led to the conclusion that one of its breakdown products is required for cell growth and division. The substance was characterized. It is called kinetin and has been classified as a phytohormone.
The technique developed by F. SKOOG proved to be ideal for the study of the regeneration capacity of callus cultures. Callus and tissue cultures can both be kept in light or in dark. Under light exposure produce the cells at the surface plastids, chlorophyll and carotenoids.
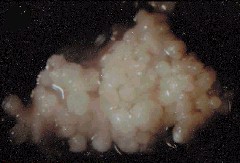
Regenerating Calli from Root Explants of Cymbidium ensifolium. The bar represents 4 mm. C. CHANG and W. C. CHANG, 1998 - Plant Cell Reports, 17, 251-255 (1998) - © Springer-Verlag
The microscopic analysis of callus tissues in the process of differentiation gives information about the regeneration capacity of plant cells.
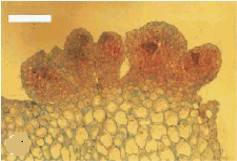
Embryoid callus with clearly differentiating areas (to the left). To the right is a microscopic preparation, a cross-section through an embryoid. The formation of meristematic tissue at part of the callus surface can clearly be recognized. The calli stem from Cymbidium ensifolium, and the bar represents 500 µm. C. CHANG and W. C. CHANG, 1998 - Plant Cell Reports, 17, 251-255 (1998) - © Springer-Verlag
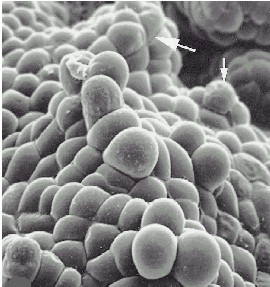 |
Scanning electron microscopy of an embryoid surface. The arrows point at secondary embryoids. The callus stems from Cymbidium ensifolium. The bar corresponds to 500µm. C. CHANG and W. C. CHANG, 1998 - Plant Cell Reports, 17, 251-255 (1998) - |
Callus cultures are useful for many purposes of pure and applied research. Among these are:
| the production of secondary plant products and enzymes by tissue cultures. | |
| their use for the synthesis of starting compounds that are subsequently modified to yield the desired product. | |
| their use as starting material for the vegetative propagation of plants. | |
| their use as basic material fore high-yield cultivars (maintenance breeding). | |
| their reverting to tissue cultures allows the conservation of virus- or fungi-free and resistant cell lineages. |
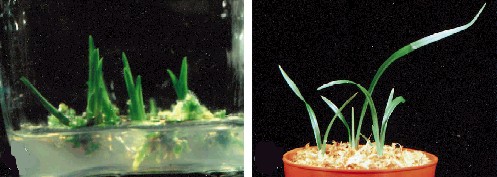
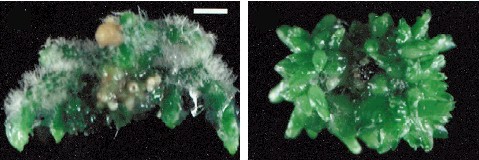
Plants Regenerated from Calli of Cymbidium ensifolium. The plant at the left is still on agar. Its roots and shoot are clearly differentiated. At the right is the same plant shown after potting. C. CHANG and W. C. CHANG, 1998 - Plant Cell Reports, 17, 251-255 (1998) - © Springer-Verlag
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de