The fact that the physiological activity follows the day’s course is known since antiquity. The observations and experiences are not restricted to humans and animals alone, but included the activities of plants (the rising and lowering of leaves, the opening and closing of flowers), too. These phenomena were experimentally studied since the 18th century. The Parisian astronom DeMAIRAN detected in 1729 that the plants’ movements remain even when they are kept in constant darkness. The German J. G. ZINN from Hamburg discovered some thirty years later that the leaves of the bean (Phaseolus coccineus) rise and lower even without the light-darkness stimulus, and that these movements are almost independent of the temperature.
C. v. LINNÉ observed that the opening and closing of the flowers occurs in a species-specific way at certain times of the day (clock of flowers, depicted in 1741 in the Horologium florae, published in Philosophia Botanica).

J. v. SACHS (1857, 1863) demonstrated that the rhythmic is influenced by two components: a hereditary one securing a rhythmically running movement, and a controlling one that fixes the beginning of the rising-lowering-cycle. His plant anatomical studies of the leaf stalk base of Oxalis carnea elucidated the joint’s structure and were the base for the characterization of the rising and lowering of leaves as a turgor movement. The movements of petioles are, in contrast, growth movements.
 The importance of the rhythmic’s autonomy was explained by R. SEMON, and later also by
W. PFEFFER (since 1907) at the beginning of the 20th
century. The final proof was found by E. BÜNNING and K. STERN (1930, at the Botanical
Institute of the Universität Jena) when they analyzed the reactions Phaseolus multiflorus
and other species showed under thermoconstant laboratory conditions and under a given light
program (changes of light and darkness). This experimental setting secured that the movements of
the plants were indeed autonomous, i.e. controlled by an endogenous rhythmic, that is itself
regulated by the extern signal light. As soon as the control is discontinued, the length of the
period diverges significantly (dependent on species and individual) from the 24 hours, so that
it has to be talked about a circadian rhythmic (E. BÜNNING, 1986).
The importance of the rhythmic’s autonomy was explained by R. SEMON, and later also by
W. PFEFFER (since 1907) at the beginning of the 20th
century. The final proof was found by E. BÜNNING and K. STERN (1930, at the Botanical
Institute of the Universität Jena) when they analyzed the reactions Phaseolus multiflorus
and other species showed under thermoconstant laboratory conditions and under a given light
program (changes of light and darkness). This experimental setting secured that the movements of
the plants were indeed autonomous, i.e. controlled by an endogenous rhythmic, that is itself
regulated by the extern signal light. As soon as the control is discontinued, the length of the
period diverges significantly (dependent on species and individual) from the 24 hours, so that
it has to be talked about a circadian rhythmic (E. BÜNNING, 1986).
Picture to the left: Professor Erwin BÜNNING on the way in the train Nordpilen to the annual Lapland-Excursion (1963) that he arranged for biology students of the University of Tübingen
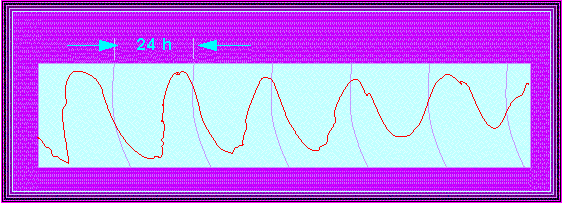
Phaseolus coccineus. Typical course of the circadian leaf movements under constant light (weak intensity). The phase shifts within six days by roughly 17 hours compared to the normal day. The length of one period is thus about 27 hours (circles in 24-hour-intervals; E. BÜNNING and M. TAZAWA, 1957).
Plants with cycles of 23 hours and others with cycles of 26 hours were identified within the species Phaseolus coccineus. BÜNNING discovered that the length of the period stays constant for several years and generations. Single plants were continuously self-fertilized and their offspring was analyzed in order to study this. 23-h-plants and 26-h-plants were crossed in a parallel experiment. The period length of the F1 generation was the average of both parental lengths, but the period length of the parents appeared again in later generations.
Lately, the studies of the endogenous rhythmic have more and more been carried out with single-celled organisms. Metabolic activities and not movements are used as a measurement of the rhythmic (production of oxygen by photosynthesis, for example). V. G. BRUCE was thus in 1972 able to prove that the length of the phase is determined by the activity of a single gene when he crossed two phyli of Chlamydomonas (one with a 24-h-phase, the other with a 26-h-phase).
The additive effect of phase-elongating genes was proven by isolation and use of further Chlamydomonas-phyli (BRUCE, 1974). If the phase of one mutant is elongated by n and that of another by m hours, then the double mutant received by crossing displays an elongation of n + m. This example shows that the endogenous rhythmic does not only occur in movements, but in the changes of metabolic ratios and other activities, too. It was necessary that animals and plants developed mechanisms in the course of evolution to measure time and to react to the change of day and night, since the day-night-rhythmic is one of the few constant parameters of our environment. It is quite striking that these phenomena have never been observed in bacteria. This is comprehensible since the length of one of their generations takes under favourable conditions and depending on the species minutes or hours. The day does not appear in the life cycle of a bacteria.
The emptying of the sporangia of some algae (and fungi) that manifest themselves on the transcriptional and the translational level as well as in the regulation of the metabolism belong among others, too, to the periodic processes of plants.
The different circadian processes of a cell are usually always in defined phase relations to each other. If the phase of one reaction is experimentally shifted by extern factors, the phases of all other reactions related to this one will shift by the same period. The effect can occur due to a mutation, since all circadian functions studied in a certain object change in the same way. We can picture this in a strictly formal sense to ourselves ith a simple model. It is based on several combined control circuits (A, B, C, D...) that are illustrated as wheels of equal size in the picture below. It demonstrates that the phases of the single control circuits are shifted against each other so that they can take on any value. Our question is now: how do organisms measure time, and how are the single activities synchronized ?
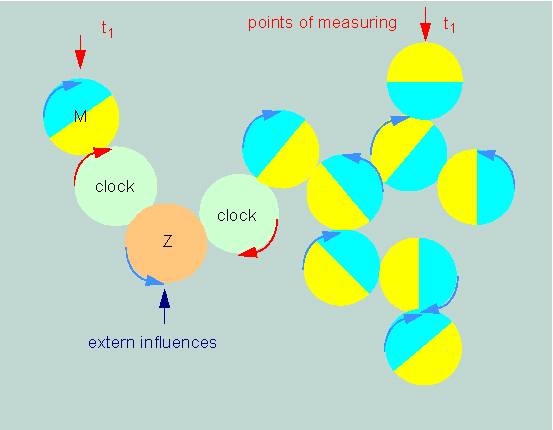
Combined Control Circuits. The periods of activity are yellow (according to F. A. BROWN, 1960)
Well-founded evidence exists that time measuring occurs with the help of a fluctuating oscillator, and not according to the principle of an hour-glass. The analogy with a watch is clear. In a watch, the energy of a spring is transmitted to a balance spring (an oscillator with a phase period in the area of seconds), from where it is passed on to the big and the little hand by gearwheels of different sizes. It does thus simultaneously control a 1-hour and a 12-hour period.
All complicated functional structures, whether they are organisms or technical devices have to be understood as controlled systems containing a large number of interconnected control circuits. The theory of a control circuit says that the actual value does always oscillate around the theoretical value. This means inevitably that oscillations of the most different frequencies, i.e. with periods of split seconds, of several seconds, minutes, hours, etc., do a priori occur in every organism. It seems that the organism selects those control circuits (reactions) with optimal frequencies from the existing circuits in order to adapt to its surrounding. It should be mentioned that not only circadian rhythms, but also a year rhythms (see, for example, photoperiodism) and, in some marine algae (and animals) a tide-dependent rhythmic are known.
We have already got to known quite a number of control mechanisms when talking about the primary metabolism. Feedback inhibition is among the fast reacting ones that controls the concentration of certain metabolites. The length of the period is in the area of seconds/minutes. In contrast, the regulation via gene expression takes hours.
As useful as the known results and models may be for the principal understanding of biological clocks, as little did they help to elucidate the actual mechanism of the circadian rhythmic. Enzymes participate in the metabolism’s regulation, and their activity is strictly dependent on the temperature. The rule of thumb says that the activity increases twice when increasing the temperature within the physiological range by 10° Celsius. In other words: the Q10-value is 2. Circadian activities, nevertheless, have a Q10 of 1 over a wide range of temperatures, which means that rhythmic activities are almost independent of the temperature. They can therefore not be explained by changing enzyme activities. No enzyme has until now been shown to belong to the ‘clockwork’. It is therefore possible that all enzyme rhythms found thus far are only expressions of peripheral rhythms, i.e. of rhythms that are controlled by the original clock. An abrupt change of temperature (supercooling) disturbs nevertheless the rhythm’s balance. An analysis of the single phases’ behaviour towards decreased temperatures showed that some parts of the cycle display hardly any or no reaction at all, while others slow down strongly. The clock does thus react to extern stimuli depending on the phase it is in.
The application of short light flashes (1-2 minutes per day) causes comparable results. The result depends here, too, on the phase in which the plants were exposed to the light. Sensitive phases are called photophilic, insensitive ones darkness-loving (photophobic).
So far, we have paid no attention to the light’s quality. The effect stays the same for wide ranges of the spectrum, but red light forms an exception.
The phytochrome system belongs to the most important light receptors of the plant as has been explained elsewhere. It is deactivated by dark red and activated by bright red light. The ratio of the two light qualities determines the amount of active phytochrome. J. v. SACHS postulated already in the last century that not the light itself, but changes in its intensity have to be regarded as the eliciting stimuli of the circadian rhythmic. A change of the spectral composition parallels the change of the daylight’s intensity (in the morning and in the evening). The last light of the day contains – in contrast to the daylight – a relatively high portion of dark red light. Dark red light does not only shorten the length of the periods, it does also cause a quick subsiding of the rhythmic. If a factor causes simultaneously short periods and a subsiding of the rhythmic, then the conclusion that it is responsible for the premature abortion of an oscillation is not far-fetched. Subsequent repetitions lead to an attenuation, and finally to an end of the oscillation.
The question for the place of light perception is another problem. It is in higher plants distinguished between cells that perceive the stimulus and cells that display rhythmic activity just like in other light-induced reactions, too. For several species, it could be shown that the light absorption occurs primarily in the epidermal cells of foliage leaves. They do often contain specifically structured cells with lens effects (ocells) specialized in light absorption. The lack of chlorophyll in the epidermis of the leaf surface may be advantageous, too, since it would otherwise compete in light absorption. Its lack results in a high sensitivity even towards small amounts of light (about 1 lux).
The mentioned phytochrome system is certainly not the only light receptor. Blue light has in many plants an even stronger effect, and is in some plants the only effective system. Such differences show that the absorbing pigments are no components of the clock, but are coupled to it.
What can finally be said about the oscillator itself? The removal of the single-celled giant alga Acetabularia’s nucleus poses hardly any problem. Parts without a nucleus remain alive for weeks and display distinct circadian rhythms (in their photosynthetic activity, for example). This means that the nucleus is not required for keeping the rhythmic active. If, nevertheless, a nucleus from a cell that was programmed with an inverse rhythmic is implanted to fragments without nucleus, then the fragment takes on the phase of the new nucleus. It seems therefore without doubt to have a directing function. Further experiments can be performed with the Acetabularia-system. It is known from molecular biology that a number of specific inhibitors for the single steps of gene expression exist. The application of Chloramphenicol and Rifampicin, for example, does not influence the rhythm, while that of Puromycin or Chloramphenicol causes heavy irregularities of the inner clock. Rifampicin inhibits transcription (the DNA-dependent RNA-polymerase), while Chloramphenicol inhibits translation of procaryotes as well as of chloroplasts and mitochondria, Cycloheximid inhibits specifically protein biosynthesis at the cytoplasmatic ribosomes, and Puromycin, finally, inhibits all sorts of protein synthesis. These inhibition experiments show that the circadian rhythmic is not influenced by transcription or the translation of chloroplasts and mitochondria, but by the translation at the cytoplasmatic ribosomes. Further experiments enhanced the assumption that the synthesis of a membrane-bound protein that blocks its own synthesis participates in the initiation of the rhythmic, and could even be the oscillator itself (H. G. SCHWEIGER, 1982). Further experiments have to prove this assumption right, and to verify its validity for other systems, too.