Right from the beginning of experimental plant physiology was the influence light exerts on the development of plants a topic of much interest. The experimental set-ups of J. v. SACHS and the late studies of W. PFEFFER and J. BONNER are among the important first approaches.
Seedlings that are cultivated in the dark are usually characterized by an intense elongation. The internodes become extremely long and leaf primordia develop, but do not differentiate. The shoots are yellowish since nearly no chlorophyll is produced, though Tradescantia albiflora, several gymnosperms and many lower plants are exceptions that synthesize chlorophyll even in darkness. A plant development that is impaired by the absence of light is called etiolation, the plants are said to be etiolated
A surplus of light like the strong UV radiation in high mountains causes drastically reduced elongation of the internodes, a decrease of the assimilating surface, often to a strongly enhanced production of anthocyanes and usually also to intensely coloured flowers.
These just shortly outlined observations were already made in the previous century and were during the last decades supplemented and backed up by the results of specific experiments. These experiments dealt mainly with the questions, which differentiation and growth processes are light-dependent, how the action spectrum looks like and which amounts of light are required.
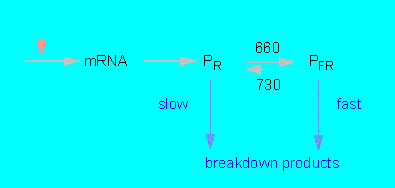
H. MOHR and his collaborators (Institut für Biologie, Universität Freiburg) analyzed a number of light-induced reactions by studying Sinapis alba seedlings. In contrast to many lower (and mainly aquatic) plants where the light-induced reactions are mostly, though not always, elicited by short-waved light (blue light), is the photomorphogenesis of higher terrestrial plants usually dependent on long light waves. Based on these observations developed H.A. BORTHWICK, S. B. HENDRICKS and their collaborators (Plant Industry Station, US Department of Agriculture, Beltsville, Md.) the concept of the red / far-red system also called the phytochrome system between 1946 and 1959.
|
All these photomorphisms can be traced back to the formation of PFR (according to H. MOHR and P. SCHOPFER, 1978) |
Inhibition of translocation from the cotyledons Increase of the surface area of the cotyledons Unfolding of the cotyledons’ lamina Development of hairs at the hypocotyl Opening of the hypocotyl’s hook Development of the primary leaves Development of mature leaf primordia Increase in the negative geotropic reaction of the hypocotyl Development of xylem elements Differentiation of the stomata within the epidermis of the cotyledons Development of super-etioplasts in the cotyledons’ mesophyll Changes in the intensity of the cell respiration Synthesis of anthocyane in the cotyledons and the hypocotyl Increase in the synthesis of carotenoids Increase in the capacity of the chlorophyll synthesis Increase in the RNA synthesis within cotyledons Increase in the protein synthesis within cotyledons Intensification of the storage fat breakdown Intensification of the Storage protein breakdown Increase in the synthesis of ethylene Acceleration of the Shibata-shift within the cotyledons Determination of the cotyledons’ capacity to photophosphorylate Modulation of the cotyledons’ enzyme synthesis |
Phytochrome
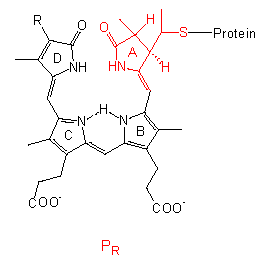
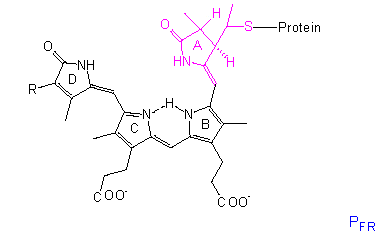
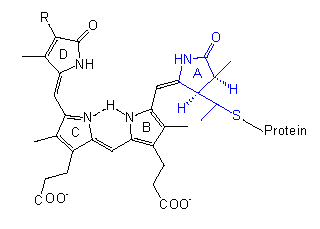
The chromophore group is a linear tetrapyrrole that differs in the conformation and absorption spectrum of its PR state clearly from its PFR state. A similar group with comparable conformational changes occurs in the bilirubins of red algae, though they bear an ethyl group instead of the vinyl group at their D-ring.
The protein is a dimer of two identical subunits with molecular weights ranging from 120,000 to 127,000 in different plant species. It is an allosteric protein. All that we known point to a structural change within the chromophore that acts like a lever and causes a conformational change of the protein. The allosteric effect results in a further amplification that changes, too, the link properties to other molecules. This may cause a cascade resulting in measurable physiological phenomenons. If phytochrome acts directly as an effector or if further molecules are involved, remains to be settled. It may also differ from case to case.
In darkness is PR produced within the cytoplasm where it is accumulated until a certain level is reached. An equilibrium between synthesis and (slow) degradation results. The transformation of PFR after exposure to red light is a fast process. PFR is extraordinarily unstable, the phytochrome level of the cell does therefore drop to one to two percent of the original value that most likely presents a new equilibrium between PR synthesis and PFR breakdown.
After darkening rises the amount of phytochrome again due to the de novo synthesis of PR. The elimination of PR is thus not just caused by a protein inactivation but by an additional inactivation of translatable mRNA. The negative feedback stops after a new exposure to light since the PFR level drops towards zero. At the same time increases the amount of mRNA (P.H.QUAIL and colleagues, University of Wisconsin, Madison).
The Phytochrome System and Its Control Possibilities
(according to P.H.QUAIL, 1984)
Further details about the mode of action were obtained by the use of genetic engineering (the mRNA was translated into cDNA and cloned; the nucleotide sequences of phytochrome-encoding genes are known) and that of monoclonal antibodies against certain domains of the protein. Even Arabidopsis thaliana, a plant with one of the smallest plant genomes, contains 5 genes encoding phytochromes. The nucleotide sequences of the single genes vary considerably. This and results obtained with other species indicate that the phytochromes belong to a remarkably variable family of proteins of at least three sub-families (phyA, phyB, and phyC). They developed before the monocots evolved. The results of the protein analyses show that two different types of photoreceptor exist: type I, the phytochrome of etiolated tissues and type II, that of green tissues. Type I occurs in etiolated tissues in large quantities and is subject to a high turnover. Type II occurs also in etiolated tissues though only in very small quantities and in its stable PR state. Since the phytochrome type I is encoded by phyA genes is it also termed phytochrome A. In contrast is the type II phytochrome rather heterogeneous. It consists of at least two polypeptide chains that are immunologically not related (and do not show any relationship to phytochrome A). The polypeptide chains of phytochromes consist of 1,110 - 1,172 amino acid residues which places them among the longest existing polypeptide chains. Each of them is linked to a chromophore and folds into two domains. It looks as if the similar chromophorous group causes the phytochromes to react to the light signal while the different protein structures allow them to forward the signal to different receptors inducing a number of different physiological reactions (QUAIL, 1991).
After the demonstration that phytochrome meshes with the control of transcription, became the search of specific DNA recognition sequences causing a light-induced transcription of DNA a major focus of interest. G. MORELLI et al. (Rockefeller University, New York, 1985) showed that a sequence of 33 base pairs is essential for the light-induced control of gene expression. It includes part of the TATA-Box ( a part of the promoter) and precedes the gene for the small subunit of Ribulose-1,5-bisphosphate carboxylase. By now has the transcription of quite a number of proteins been shown to be light-induced, among them is the chlorophyll a/b-binding protein, the a-subunit of ATP synthethase, the 32-kDa protein of photosystem II, the chalcon-synthethase, and several more (SIMPSON and HERRERA - ESTRELLA, 1990). Depending on the respective gene contained the DNA both upstream and downstream (i.e. at the beginning of a transcription unit and at its end) nucleotide sequences that are necessary for the light-induced control. They regulate the amount of produced mRNA transcripts (GILMARTIN et al., 1990).
Phytochrome has been localized within the cell plasma, the nucleus and the plastids by indirect immunofluorescence. Not all cells contain the same amount. In the epidermis, for example, occurs phytochrome nearly exclusively within the guard cells.
Phytochrome has a part in the induction of chloroplast rotation within the thread-like green alga Mougeotia. It is distinguished between the weak light and strong light position of the laminiform chloroplast (epistrophe, oblique position). The chloroplast movement is an intracellular movement that varies from cell to cell, since each cell has its own light perception. The information is not shared with other cells. Even within one cell is the movement of the single chloroplast sections autonomous as is proven by partial light exposure of a cell.
W. HAUPT (Botanisches Institut der Universität Erlangen, 1970) used micro rays of polarized light to scan cells part by part. He could thus show that the phytochrome of the cells he studied was localized at the cell’s periphery (most likely within the plasmalemma) and was oriented in a certain way.
The chloroplast reacts to the increase in PFR. During its generation from PR do the photoreceptors turn by 90 degree. PR is oriented in parallel to the cell surface, PFR vertically. The chloroplast does thus always turn away from areas with high concentrations of PFR.
How the movement itself is performed is unknown. It looks as if actin is involved, but how the light perception is transformed into kinetic energy or how the actin filaments are attached to the chloroplast remains unsolved.
The orientation of phytochrome molecules at the cell periphery is not restricted to Mougeotia alone. A similar phenomenon causes the phototropic reaction of fern chloronema. On the other hand is phytochrome, especially in algae, not the dominating sensory pigment. The deeper the water the lower the portion of long-waved light, consequently displays blue, short-waved light a stronger influence on algae than that of longer waves. The phytochrome system with its adaptation to long-waved light has no advantages for the alga.
Algae and other organisms seem to have four physically different concepts for the light perception during chloroplast movements.
A number of physiological processes like the generation of anthocyane are only activated or run with maximal capacity after a longer exposure to light. The action spectrum covers a wide range of light wave lengths, more, actually, than you would expect according to the absorption spectrum of phytochrome. Obviously is more than one sensory pigment activated.
K. M. HARTMANN (Institut für Biologie, Universität Freiburg) showed as soon as 1966 that the growth of lettuce seedlings is not influenced by the subsequent exposure to light of the wave lengths lambda = 658 nm and lambda = 768 nm, while the exposure to both wave length at the same time increases growth. Everything points at phytochromes as light receptors for these reactions. To explain the mode of action was it assumed that further phytochrome states PRX, PFRX, PRX’, and PFRX’ exist beside PR and PFR and can reversibly (directly or indirectly) be transformed into each other. They are in equilibrium with each other within the cell. It looks as if energy was transferred between the sensory pigments (or their different activity states) thus causing a strong modulation of the sensitivity towards light. This amplifies the original light signal. Strong indications for further photoreceptors for blue and UV light exist. Indeed have such receptors been identified (cryptochrome, a blue-light receptor, and the UV-B-photoreceptor). It was shown, too, that both modulate the phytochrome system. Their existence enables the plant to react exactly to the light conditions of its habitat. In algae stimulates blue light the synthesis of carotenoids, and of chlorophyll and the breakdown of glucose. In some marine species is the development of thylacoids influenced, while blue light regulates the endogenous rhythm of Acetabularia.
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de