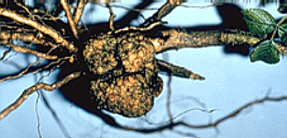
Plant tumors are elicited by either bacteria, viruses or genetic factors. The tumors develop often after hybridization of related species belonging to the genera Brassica, Bryophyllum, Lilium, Lycopersicon, and Nicotiana. Hybridizations of Nicotiana glauca x Nicotiana langsdorfii are characterized best. Their occurrence seems to be the expression of a lacking co-operation between the two parental genomes. In the following, we will exclusively discuss crown galls induced by a plasmid that is transmitted by Agrobacterium tumefaciens (J. SCHELL and collaborators, University of Gent/ Belgium, 1974, and Max-Planck-Institut für Züchtungsforschung, Germany).
The transformed cells (tumor cells) and the calli developing from them are less demanding than normally differentiated cells. They are, for example, not dependent on an extern supply of auxine (they are ‘auxine autotrophic’). Auxine autotrophy occurs sometimes also in normally differentiated cells causing so-called ‘habituated’ cells.
Crown galls caused by Agrobacterium tumefaciens have been shown to occur in more than 100 dicot genera. Monocots develop no tumors, though some of them become infected.
A second species of bacteria, Agrobacterium rhizogenes induces root tumors in a number of plant species. Here, too, a plasmid (the R1-plasmid) plays an important part.
Two types of crown galls exist: the so-called teratomes are tumors producing shoot- or leaf-like structures or root-shaped excrescences continuously. The other type of crown gall shows no remarkable differentiation.
It is solely the plasmid (Ti-plasmid, tumor-inducing plasmid) that determines which type of tumor develops. After the cell has been infected with the bacterium, the plasmid enters the plant cell and is incorporated into the plant genome. The plasmid is relatively large, and a number of different plasmid types with molecular weights between 100 and 170 x 106 exist. The average weight is 112 x 106, the average length 54 µm. Only a small portion of the plasmid – the T-DNA – is responsible for the induction of the tumor. Genes that are localized on the plasmid can be expressed both in the bacterium and the plant cell, i.e. the plasmid contains procaryotic and eucaryotic promoters. This proved to be a prime feature for using it as a vector smuggling foreign genes into the plant genome. The plasmid became an ideal aid for the genetic engineering of plants. The lifespan of bacteria within plant cells is rather short, but the neoplastic state (the tumor state) of the plant cells is stable and stays intact even after the bacteria have died. The bacteria themselves are consequently required only as a transport aid for bringing the plasmid into the plant cell. The now transformed, new tumor cells accumulate ‘rare’ amino acid derivatives: octopine or nopaline. Both derive from arginine.
The enzymes required for the synthesis and the breakdown of these rare amino acid derivatives are encoded by a plasmid. The plasmids do either metabolize octopine or nopaline. Octopine and nopaline are produced within the plant cell, but their breakdown takes place within the bacteria. It looks as if the bacteria had thus secured a safe source of food while living in the plant cell.
During the last years, Ti-plasmids have been thoroughly studied by two different approaches:
The structure of the plasmids is studied and their genes are characterized. The T-region (T-DNA) is of special interest, since
it is tried to alter the Ti-plasmid in order to get a vector for the transfer of foreign genes into the plant cell. Segments that seem not to be required for genetic engineering are removed. Combinations of Ti- plasmid fragments with segments of other bacterial plasmids increase the host range. SCHILPEROORT and his colleagues of the University of Leiden, Belgium, for example, have incorporated the Ti-plasmid into Rhizobium-species. Rhizobium is the nitrogen-fixing nodule bacterium of leguminosae. Rhizobium-species have a mechanism of their own that allows them to enter plant cells. Some Rhizobium species (though not all!) acquire the ability to induce tumors after having taken up the plasmid. The genera Agrobacterium and Rhizobium are closely related. Both are gram-negative soil bacteria.
In addition to the genes required for the production and the breakdown of octopine and nopaline, the T-DNA contains segments, that
- control growth and differentiation of the plants,
- have a direct influence upon the plant’s metabolism.
In plant cells, RNA-polymerase II transcribes the plasmid’s DNA. Neoplastic, plasmid-transformed cells are hormone autotrophic. It was thus looked for genes with products that unbalance the hormone system of the plant. Genetic analyses showed that the T-DNA of all tested types of Ti-plasmids contains six conservatively structured transcription units, though one or the other unit may lack in mutants.
It has been mentioned at the beginning, that many tumors are teratomes, i.e. their cells can differentiate alternatively into shoots, roots, or both. The products of the T-DNA genes 1 and 2 inhibit a differentiation into a shoot and induce the production of roots. Callus cultures of normal (auxine-heterotrophic) cells display a similar effect after auxine has been added to the nutrient medium. Gene product 4 alone is enough to induce a tumor, it is not dependent on the activity of the other gene products. In summary, it can be said that genes 1, 2, and 4 together shift the auxine / cytokinin balance of transformed cells, thus causing the largely undifferentiated (neoplastic) state of the cells. The respective gene segments were brought into suitable plasmids expressed in Eschericia coli. Gene product 2 turned out to be an aminohydroxylase catalyzing the following reaction:
Indole–3–acetamide (IAM) > Indole–3–acetic acid (IAA)
The same enzyme catalyzes the terminal step in plant auxine biosynthesis.
Gene product 4 is required for the synthesis of cytokinin. The mutants mentioned above, that have tumors developing only roots or shoots are characterized by the lack of the respective hormone (G. SCHRÖDER, S. WAFFENSCHMIDT, E. W. WEILER, and J. SCHRÖDER, Max-Planck-Institut für Züchtungdforschung, Cologne, and Institute for Biology, Universität Freiburg, 1984; I. BUCHMANN, F.-J. MARNER, G. SCHRÖDER, S. WAFFENSCHMIDT, J. SCHRÖDER, 1985).
|
|