| Quiz Me! |
Chemicals in the Cell II |
||
| Lecture Index | |||
| Course Index |
Last revised: Friday, September 10, 1999
Reading: Ch. 3,4 in textNote: These notes are provided as a guide to topics the instructor hopes to cover during lecture. Actual coverage will always differ somewhat from what is printed here. These notes are not a substitute for the actual lecture!Copyright 1999. Thomas M. Terry
Strong and Weak Chemical BondsCovalent Bonds = strong bonds (50-100 kcal/mole)
- shared electron pairs
- Exs: CH 4 , H 2 O
- possible to have single bonds, double bonds, even triple bonds
- Exs: H-H (single bond); O=O or O=C=O (double bond); N
N (triple bond)
View double and triple bonds
- Number of bonds possible depends on valence
Ionic Bonds (strong in crystals; weak in water: ~ 5-10 kcal/mole)
Ionic Bonds
- Ions can react to form compounds such as: Na + and Cl - ----> NaCl(table salt)
- Actually never just two atoms involved, usually millions or billions, alternating: Na + Cl - NA + CL - NA + CL - NA + CL - NA + CL - etc., in 3 dimensions. These are called salts
- Biomolecules contain a number of + and - charged groups which can form ionic bonds, such as carboxyl (-COO - ) and amino (-NH3 + ) groups.
- Ionic bonds are quite strong in the absence of water.
- However,once in water, ions become hydrated by shell of surrounding water molecules, ionic forces become weak (3-10 kcal/mole)
- In hydrophobic environments (such as inside a folded protein), ionic bonds can become quite strong (50 kcal/mole or more).
Hydrogen Bonds (weak: 3-5 kcal/mole)
- Hydrogen bonds result from electrostatic attraction between electronegative atoms (such as O or N) and a hydrogen atom that is bonded covalently to a second electronegative atom.
- Examples:
- N-H --- O=C -
- -O-H----- O=C -
- Hydrogen bonds are weak bonds, typically about 3-5 kcal/mole
- View animation of Hydrogen bond formation at The Biology Place
- View Hydrogen bond formation in Water
Van der Waals bonds (weak: 1-2 kcal/mole)
- Van der Waals bonds are the weakest of all bonds.
- As any two atoms approach each other, electron clouds begin to overlap. Creates a situation known as "induced dipole". One electron "pushes" other to opposite side of its atom, so momentarily there is a slight electron deficit, therefore a slight + charge to attract the first electron's - charge. This situation oscillates back and forth between the two atoms, creating a very slight attractive force, ~ 1 kcal/mole = "Van der Waals" bond
- VDW attraction is very local, only occurs when atoms are touching. If many atoms are involved, the individual forces add up, can be very strong.
- Ex 1: antibody and antigen reaction
- Ex 2: insect walking on ceiling molds its footpads to atoms of ceiling, creates enough VDW attraction to counteract gravity.
"Hydrophobic bonds" (weak: 1-5 kcal/mole)
- Non-polar molecules mixed with water don't dissolve (e.g. oil slick on water). Why?
- Water is held together by hydrogen bonds. If nonpolar molecule is inserted into water, would have to break the ordered lattice of water molecules held together by H bonds. But this would require energy -- can't happen spontaneously. Instead, nonpolar molecules (or parts of molecules) will aggregate to avoid water. A similar situation occurs in parts of many proteins.
- This is an example the role ofEntropy, a concept related to the measurement of disorder. We will explore entropy in more detail in conjunction with the study of Energy in Ch. 6. Entropy and Enthalpy (heat energy) are both involved in determining the overall Free Energy of a system.
- Hydrophobic bond is not an attractive force, rather a lower energy state than would occur if these molecules were dissolved in water, resulting in an increased disordering of the lattice of ordered water molecules. So it has a measurable energy comparable to attractive force.
- View Hydrophobic bonds
Aqueous Chemistry
- All life processes occur in water. Some cells can survive without water, but only as dormant stages. Water is critical to life. Why?
Properties of Water
- Results from the strong polar nature of water
- Excellent solvent -- will dissolve most substances (except for large non-polar molecules)
- High heat capacity -- as heat is added, it is "soaked up" by H-bonds,contributes to slight loosening of attraction between water molecules. Can soak up lots of heat before individual molecules let go of each other, vaporize as steam.
Moles and Solutions
- All biological laboratory work involves solutions.
- Weight of individual atoms is too small to weigh. One proton weighs 1 dalton, a tiny fraction of a gram.
- Practical convention: instead of weighing substances in daltons, use grams. To keep track of numbers of molecules, use Mole concept.
- Mole = amount whose weight in grams is numerically equal to molecular weight of a substance.
- Example 1 : Methane (CH4) has formula weight of (12x1 + 1x4) = 16.
- To get one mole of methane, weigh out 16 grams.
- Solution containing 1 mole of a substance in 1 liter of water is 1 molar. If solution contains only 1/10 mole, is 1/10 molar, or 0.1 M.
- Example 2 : to make a solution of 0.01 M glucose sugar, first calculate molecular weight of glucose: C 6 H 12 O 6
6x12 + 12x1 + 6x16 = 180- To make 1 molar glucose, add 180 g/liter. To make 0.01M, add 1.8 g/liter.
Acids,Bases, and pH
- Water ionizes very slightly. H 2 O --> H + + OH -
- H + = hydrogen ion
- OH - = hydroxyl ion
- View movie showing water dissociation ( protected)
- Concentration of in pure water is only 10 -7 M/liter
- ACID = any substance that dissociates (ionizes) to produce H + ions
- BASE = any substance that can accept H + ions
- When pure water produces H + it always produces equal # of OH - ions; no net change in amount of H + ; but other acids change amount of H + in solution.
- Strong acids: dissociate completely. Ex: HCl --> H + + CL -
- Weak acids: dissociate partially. Ex: acetic acid
CH 3 COOH --> CH 3 COO - +H +
View dissociation of phosphoric acid
- pH : a way of expressing concentration of in familiar numbers.
- Typically defined as the negative logarithm of H + concentration. For pure water, pH = 7, since conc. is 10 -7 M/liter
Some common pH values in biological systems:
stomach acid 1.3-3 lemon juice 2.1 coke, orange juice 3 tomatoes 4 saliva 6.5 distilled water 7.0 blood 7.4 baking soda 8.4 bleach 11.9 oven cleaner 13
- Note: variation by 1 pH unit is 10x difference in H + conc.
- Importance of pH: cell interiors are typically around pH 7 (7.4 in humans).
- Variation of pH of 1 pH unit is lethal; changes proteins (more on this later),causes death.
- How do organisms regulate pH?
Buffers
- Buffers are mixtures of acid and corresponding base, substances that only ionize partially, can "soak up" excess H + ions.
- Example : H 2 CO 3 --> HCO 3 - ( bicarbonate ion ) + H +
HCO 3 - --> CO 3 = + H +- If H + is added to solution, gets soaked up by forming more HCO 3 - , more H 2 CO 3
- In blood, bicarbonate is the common buffer. Other examples: phosphate salts,Tris, others (find tables for lab use).
- Buffers typically most useful over a 2 unit pH range.
Organic Compounds
- All molecules containing C (except CO 2 ) are organic compounds. Millions of examples.
- Since C has valence of 4, can add many possible other atoms to C, generate enormous number of different molecules.
- Ex: CH 4 , CH 3 OH, H 2 C=O (just some 1 C compounds)
Functional Groups
- Certain combinations of atoms (e.g., OH) have characteristic properties different from their individual atoms. Recognizing a handful of these functional groups is very useful for chemists and biologists.
Group Name Role 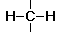
hydrocarbon insoluble in water nonpolar does not form H bonds electrons equally shared between H and C 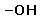
hydroxyl ( -OH) (Click to view figure) water soluble; forms H bonds found in alcohols and sugars 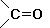
carbonyl ( -C=O) (Click to view figure)
Try interactive quiz on hydryoxyl and carbonyl groups at The Biology Place. forms H bonds found once in all sugars If bonded to Hydrogen atom = aldehyde group (H-C=O) If not bonded to H atom = keto group (-C=O) 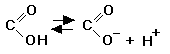
carboxyl ( -COOH) (Click to view figure)
View animation of carboxyl group ionization at The Biology Place
Try interactive quiz on carboxyl and other O-containing groups at The Biology Place. good acceptor of H bonds acid; often ionized to COO - 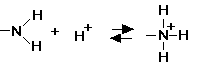
amino ( -NH2) at the Biology Place forms H bonds base; often ionized to NH3 + 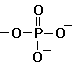
phosphate ( -PO4=) (Click to view figure) forms H bonds; very soluble always ionized 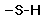
sulfhydryl ( -SH) (Click to view figure) forms weak H bonds two -HS groups can react to form S-S (disulfide) bonds used to crosslink polypeptides
Isomers
- Because Carbon can form so many bonds, there are a situations in which molecules with the same elemental ratios (e.g. C 6 H 12 O 6 ) can occur in different spatial arrangements. These are called isomers .
- There are 3 common types of isomers:
- structural isomers differ in the arrangement of covalent bonds.
Example: see text Fig. 4.6- geometric isomers always involve arrangement about a double bond. Double bonds are rigid and do not allow the joined atoms to rotate freely. As a result, two isomers are always possible if different groups are attached around a double bond.
Example: molecules (a) and (b) are not the same, even though they have the same functional groups attached to the same Carbon atoms. Surprisingly, enzymes can easily recognize this difference and may bind tightly to one molecule and not at all to the other. Molecular shape is important in biology.
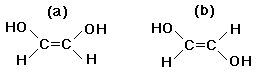
- enantiomers (left- and right- handed isomers) are isomeric forms that can occur whenever a Carbon atom is bonded to four different functional groups. The four covalent bonds formed by Carbon do not lie in a plane, but form a tetrahedron in three dimensional space. (See text Fig. 4.6). As a result, there can occur two possible configurations of the groups attached to a C atom. These are mirror images of each other, and, like mirror images, cannot be superimposed no matter how they are rotated.
Review enantiomers at The Biology Place
View molecule with single asymmetric Carbon atom
View L- and D- amino acid forms
- The bottom line : Because so many types of isomers are possible,biological molecules can occur in an amazing variety of forms. A formula as simple as C 6 H 12 O 6 can refer to more than a dozen different molecules, each recognizably different from the others by cells and by chemists.
Take a self-quiz on this lecture
Take an additional self-quiz on this lecture from the Biology Place.
Return to Lecture Index
Return to Biology 107 Index page