| Quiz Me! |
Metabolism: How Cells acquire Energy |
||
| Lecture Index | |||
| Course Index |
Last revised: Tuesday, October 5, 1999
Reading: Ch. 9 in textNote: These notes are provided as a guide to topics the instructor hopes to cover during lecture. Actual coverage will always differ somewhat from what is printed here. These notes are not a substitute for the actual lecture!Copyright 1999. Thomas M. TerryUse of ATP to store Energy
- Certain molecules widely used as cellular chemical energy: ATP, GTP, a few others
- Structure of ATP
- Interact with ATP molecule (Requires Chime plug-in)
- Think of ATP as: P~P~P-sugar-base. Each ~P bond ("squiggle-P") has high free energy content.
- ATP Hydrolysis: ATP + H2O ---> ADP + Pi
Go' = -7.3 Kcal/mole -- very exergonic
- ADP Hydrolysis: ADP + H2O ---> AMP + Pi
Go' = -7.2 Kcal/mole -- very exergonic
- Note: these rxns don't actually occur in cell -- no enzymes to carry them out!
- Once ATP is used up, must be regenerated.
- ATP Synthesis: ADP + Pi ---> ATP
Go' = +7.3 Kcal/mole -- very endergonic!
- Reaction cannot happen as written: needs some source of energy
- are the "big spenders" -- lots of energy released.
- Typical cell "trick": use energy from oxidation reactions to create ~P bonds, transfer to ADP to make ATP. Substrate-level phosphorylation (SLP).
- Another trick: use energy from oxidations to separate H+ ions across a membrane = proton gradient. Couple this energy to ATP synthesis. Chemiosmotic phosphorylation (also called oxidative phosphorylation).
- A third trick: harness light energy to make a proton gradient. Couple this energy to ATP synthesis. Photophosphorylation.
Biological Oxidations
- Redox reactions --- often associated with Energy transfer in cells
- Definition: Loss of Electron = Oxidation. Gain of Electrons = Reduction
(LEO the lion says GER)- Examples:
- Fe++ ---> Fe+++ + e- -- occurs in cytochromes
- succinic acid ----> fumaric acid + [2H] -- a dehydrogenation reaction
- oxidation step
note: 2H = 2 H+ + 2 e-
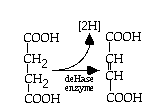
- reduction step NAD+ (red) + 2H+ + 2e- ----> NADH (ox) + H+
- oxygen rarely involved
- Redox reactions always occur in pairs; as one substance is oxidized, another is reduced.
Redox Carriers
- NAD+ = common redox carrier; alternately get reduced, then oxidized.
- View structure of NAD+; also see text Fig. 9.4
- Very little NAD+ in cell; continually recycled from (reduced) to (oxidized) state and back
- Other redox carriers:
- FAD -- carries 2H
- Ubiquinone (Coenzyme Q) -- carries 2H
- Heme groups (in cytochromes) -- carries single electron
- View diagram of other redox carriers
- Example (from TCA cycle) -- occurs during breakdown of glucose to pyruvate
Embden-Meyerhof (glycolysis) pathway
- Most common pathway for energy metabolism
- 11 enzymes in cytoplasm of cells (not in mitochondria!)
- View animation of Glycolysis (from Cornell Univ. -- requires Shockwave plug-in)
- View diagram of Glycolysis (from University of Virginia Bio 121 course)
- 3 stages:
- preparation phase (6-carbon phase); add two phosphate groups from ATP (net cost = 2 high-energy phosphate groups, or 2 ~P), then split into two 3-carbon molecules
- oxidation phase (energy release):
- oxidize glyceraldehyde-3-phosphate.
- Lots of energy is released (~ 100 kcal).
- Some energy trapped as NADH (reduced).
- Some energy used to push inorganic phosphate (Pi) to the 3-C molecule, forming 1,3-bis-glyceric acid
- Rest of energy is released as heat.
- Energy harvest phase: when phosphate groups are configured to high energy state, they can be passed to ADP ----> ATP. This happens twice for each 3-C molecule. Since one glucose produces two 3-C molecules, total yield at this stage is 4 ATP. The resulting 3-C molecule is pyruvic acid, or pyruvate.
- Net result:
- glucose + 2 ADP + 2 Pi + 2 NAD+ ---> 2 pyruvate + 2 ATP + 2 (NADH + H+)
- Note: total energy of glucose available by aerobic oxidation = 688 kcal/mole.
- Total energy yield of 2 ATP = 2 x 7.3 = 14.6 kcal/mole. This is ~ 2% efficient compared to possibility for total oxidation: -- 98% of energy potentially available in glucose is not available to cell.
- View animation summarizing glycolysis ( protected)
Problem: what do with electrons removed by oxidation reactions?
Solution 1: Fermentation
- very little NADH in cell
- Must get rid of electrons and protons, get back NAD+
- bucket-brigade analogy
- Where to get rid of electrons?
- Pyruvate is product of glycolysis
- Many cells use pyruvate as terminal electron acceptor
- waste products dumped out of cell
- Lactic acid fermentation (1 step)
- pyruvate + NADH -------> lactic acid + NAD+
- found in many bacteria: lactic acid bacteria, also in some protozoa, water molds, even human skeletal muscle
- Responsible for souring of milk products ---> yogurt, cheese, buttermilk, sour cream, etc. Excellent keeping properties.
- Alcoholic fermentation (2 steps)
- Two successive reactions:
- pyruvate --------> acetaldehyde + CO2
- acetaldehyde + NADH -------> ethanol + NAD+
- Found in many fungi, yeasts, some bacteria.
- Very important in human applications. Bread, alcoholic spirits.
Solution 2: Respiration
- Respiration = use of external electron acceptor. Usually oxygen.
- Respiration is localized within mitochondria
- 3 steps in respiration
- Transfer electrons and protons in NADH to electron transport system (ETS) in mitochondrial membrane
- Build up a proton gradient using ETS
- Use ATP synthase to convert energy of proton gradient into ATP formation
- Electron Transport
- Electron transport system (ETS) = membrane-bound pathway transferring electrons from organic molecules to oxygen.
- ETS carriers:
- Flavoprotein (FMN)
- Iron-sulfur protein (Fe S)
- Ubiquinone (Q)
- cytochrome b
- cytochrome c1
- cytochrome c
- cytochrome a
- cytochrome a3
- ETS consists of 4 spatial complexes, connected by mobile carriers (Coenzyme Q, cytochrome c) that shuttle between complexes in membrane
- electrons are passed from carrier to carrier inside the membrane
- protons are moved from inside to outside of membrane. This builds up a proton gradient.
- View organization of electron transport chain ( protected)
- View Animation of electron transport chain
- View schematic diagram of mitochondrial inner membrane enzymes (from University of Virginia Bio 121 course)
- Net result: electrons enter ETS from carriers like NADH or FADH, wind up at terminal cytochrome a3, get attached to oxygen.
- Proton gradient
- Chemiosmotic hypothesis (Peter Mitchell, 1961). As electrons flow through ETS, protons (H+) are moved from inside to outside of the membrane.
- View diagram of chemiosmotic electron flow ( protected)
- This builds up proton gradient; since + charges are removed from inside of cell, - charge remains inside, mainly as OH- ions.
- pH just outside membrane can reach 5.5, pH just inside membrane can reach 8.5 ---> difference of 3 pH units, or 1000x concentration differential of H+ across membrane.
- This represents potential energy stored = protonmotive force.
- Membrane is basically impermeable to protons, so gradient doesn't get squandered away by leaky reentry.
- ATP synthase
- ATP synthase = protein complex (also "lollipops, F1 complex, mitochondrial ATPase)
- View electron micrograph showing ATP synthase particles
- The only protein that allows proton entry to mitochondrial matrix.
- As protons push in through channel, ADP + Pi ---> ATP.
- This is called chemiosmotic phosphorylation (assuming chemiosmotic hypothesis or oxidative phosphorylation (makes no assumption about mechanism).
- View animation of proton gradient formation and ATP synthesis
Inhibitors of Oxidative Phosphorylation
- Several chemicals can block electron transfer in ETS, or transfer of electrons to oxygen. All are strong poisons. Some examples:
- Carbon monoxide -- combines directly with terminal cytochrome oxidase, blocks oxygen attachment
- Cyanide (CN-) binds to cytochrome iron atoms, prevent electron transfer.
Krebs (Citric acid, TCA) cycle: further catabolism of pyruvate
- Krebs cycle enzymes are localized in the matrix of the mitochondrion (a few of the enzymes are carried in the inner mitochondrial membrane).
- Note that glycolysis enzymes (above) were all found in cytoplasm, not in mitochondrion.
- View Overview of Krebs cycle
Formation of acetyl-CoA
- Oxidation of pyruvate (3-C) + NAD+ -------> Acetyl-CoA (2-C) + CO2 + NADH
- Note: Acetyl-CoA can also be produced by breakdown of lipids or certain amino acids -- important focal point of central metabolism
- View formation of Acetyl CoA from pyruvate ( protected)
Net effects of TCA cycle (see text Fig. 9.11)
- View details and animation of TCA cycle (from University of Virginia)
- To start cycle: Acetyl-CoA (2-C) + oxalacetate (4-C) -------> citric acid (6-C)
- Subsequent steps:
- Convert citrate to isocitrate (still 6-C)
- Oxidize -------> alpha-ketoglutarate (5-C) + CO2 + NADH
- Oxidize -------> succinyl-CoA (4-C) + CO2 + NADH
- SLP reaction: succinyl-CoA (4-C) + GDP -------> succinate (4-C) + GTP (Note: GTP can be interconverted with ADP to form ATP)
- Oxidize -------> fumarate (4-C) + FADH2
- convert fumarate to malate, oxidize again -------> oxalacetate (4-C) + NADH
- Net yield for one turn of TCA cycle (3-C):
Acetyl-CoA (2-C) + 3 NAD+ + FAD -------> 2 CO2 + 3 NADH + FADH2 + ATP- Net yield for one molecule of glucose, converted to 2 pyruvates, then to 2 Acetyl-CoA and thence to CO2 via TCA cycle, with all NADH and FADH converted to ATP by respiration:
- 1 glucose + 38 ADP + 38 Pi -------> 6 CO2 + 38 ATP
- View animation summarizing Krebs cycle ( protected)
- Note: 2 of the NADH molecules are formed in cytoplasm during glycolysis. In order to import them into the mitochondrial matrix, so they can be further oxidized by the electron transport system, they need to be carried across the mitochondrial membrane by an active transport carrier. This "costs" 1 ATP per NADH. Thus the final net yield of ATP per glucose is 36, not 38 ATP.
- View net energy gain from glycolysis and Krebs cycle ( protected)
- TCA cycle completes the oxidation of carbons in pyruvate to most oxidized form (CO2); removes electrons originally in C-H bonds to electron carriers NADH and FADH for use in respiration machinery.
- Efficiency of respiration: almost 40% of energy initially present in glucose is captured as ATP; the remainder is liberated as heat.
Take a self-quiz on this topic
Return to Lecture Index
Return to Biology 107 Index page