Lecture 21
Fluorescence in photosynthesis
|

Fluorescence from photosystem II
In green plants, the fluorescence under physiological conditions comes
from the photosystem II linked antenna, and provides a useful means of
assaying various physiological parameters which affect photosystem II function.
The fluorescence reflects the competition between
several pathways for the excitation captured in the antenna. Under
low-light conditions when photochemistry occurs with its maximal efficiency,
excitation is passed mainly to the photoreactions (with an effciency >90%).
When the photochemical traps are closed, excitation is lost by a competition
between fluorescence and non-radiative dissipative pathways, the latter
converting the energy to heat.
Because the fluorescence yield varies inversely with the fraction of open reaction centers, it provides a useful tool for investigation of photosynthetic processes. The yield can be assayed either from the fluorescence during continuous illumination, or from the fluorescence yield of a weak (<1% actinic) measuring flash. The later approach can be used to measure the reopening of the reaction center after a strong actinic flash.
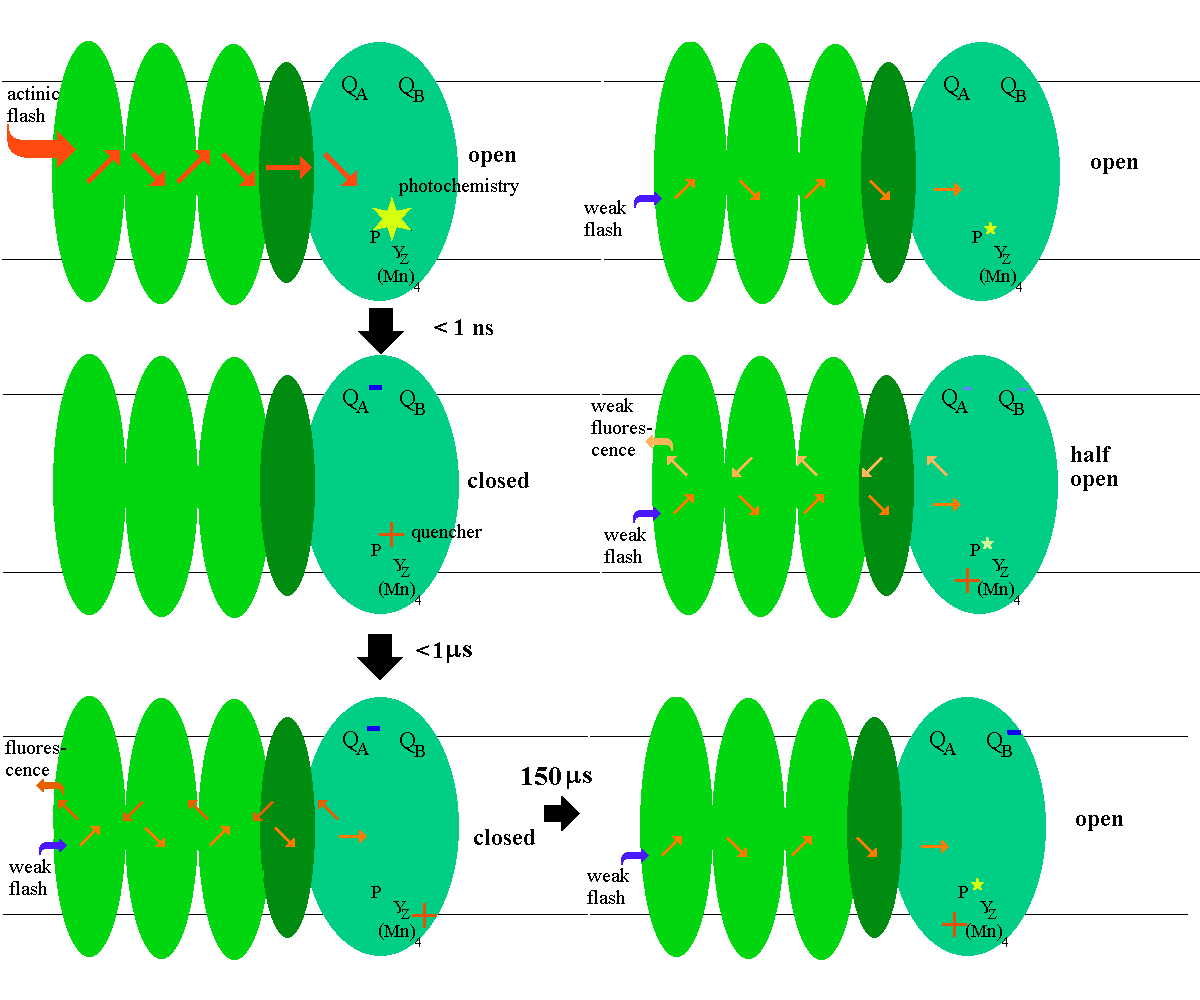
Fluorescence kinetics to assay the reduction of plastoquinone by photosystem
II
On illumination by continuous light, the fluorescence of a photosynthetic
system rises from a minimal level (F0) by a complicated kinetics,
which depends on the physiological state of the system.
The fluorescence yield is determined by a number of factors:
- Fluorescence changes associated with photochemical quenching (qQ-quenching)
in open reaction centers. Reaction centers ‘close’ as the acceptor, QA,
becomes reduced (either on reduction of the plastoquinone pool or because
the photon flux exceeds the capacity for reoxidation), and the fluorescence
rises.
- Centers can also become ‘closed’ through oxidation of the primary donor,
but in this case fluorescence remains low because P680+ acts
as a static quencher. It has generally been assumed in measurements with
intact plants that this process is negligible, but this has not been adequately
tested.
- The oxidized plastoquinone of the pool is a quencher, which has been
largely ignored in most previous work, but has recently been shown to contribute
significantly to the quenching under conditions where PSII efficiency is
high.
- Formation of Chl triplets causes a quenching, which can be significant
at high light intensities.
- Fluorescence lowering, or qE-quenching associated with the
dumping of excess excitation energy under conditions of high light, which
follows the generation and decay of low lumenal pH.
- Fluorescence lowering is modulated by formation of zeaxanthin and/or
antheraxanthin, and may require that these de-epoxidation products of violaxanthin
are present. The de-epoxidase of the lumen shows a strong dependence on
pH, with increased activity as the pH falls below ~5.5.
- Irreversible quenching associated with photoinhibition (qI)
which occurs when PS II is damaged, especially on the donor side.
- The redox state of the acceptor pools in intact systems reflects the
state of activation of the assimilatory pathways, and also the effect of
the back pressure from the proton motive force (back pmf) on the differential
rate of filling and emptying of the quinone pool.
- The redox state of the donor side reactions is also strongly dependent
on the lumenal pH, since protons are generated in the lumen is a product
of water oxidation, and their activity enters directly into the mass action
equation. Because the redox potentials of the partial reactions are not
much lower than that of the P680/ P680+ couple at neutral pH,
the equilibrium constants of the donor side are modulated so as to favor
oxidation of P680 as the internal pH drops.
- State 1 - 2 transitions lead to changes in fluorescence associated
with changes in absorption cross-section distribution between the photosystems.
Fluorescence induction
The yield of fluorescence (fF)
during the initially phases (the induction of fluorescence) on continuous
illumination, depends mainly on the state of the plastoquinone pool, and
the competition between electron delivery to the pool, and exit from the
pool. Delivery is determined by the light intensity, and the functionality
of photosystem II. In a physiologically competent system illuminated at
intensities in the ambient range, we can consider three conditions which
exemplify useful experimental regimes.
- The system inhibited by DCMU. When DCMU is present in excess
to inhibit reduction of the quinone pool through the QB-site
of photosystem II, the fF rises from
the F0 level to a maximal level (Fmax) as QA
is reduced through the photochemistry of the reaction center. The rate
depends on light intensity. The value of Fmax, DCMU depends
on the state of the pool, since Q (but not QH2) is a weak quencher.
Fmax can be as much as 30% higher with the pool reduced than
with the pool oxidized. The area over the induction curve reflects the
stoichiometry of the acceptor pool; with DCMU present, this is 1, because
each photosystem II has one primary acceptor, QA.
- Electron exit from the quinone pool inhibited. This can be achieved
by use of inhibitors of the bf-complex, by destruction of plastocyanin,
or by excluding photosystem I acceptors. In these conditions, electrons
fill the acceptor pool, and the fF
rises to the Fmax level seen with DCMU when the pool is reduced.
The area above the rise curve reflects the size of the acceptor pool, which
can be normalized by comparison with the area above the curve in the presence
of DCMU to get a value for the stoichiometry with respect to photosystem
II.
- Electron acceptor present. When an acceptor of electrons from
any point in the chain after photosystem II is present, the induction curve
is modified by the competition between photochemical reduction and oxidation
by acceptor. The shape of the induction curve, the maximal level of fluorescence,
and the detailed kinetics, depend ina complicated fashion on the interplay
between the factors listed above.
Kinetics of fF change on flash
illumination
When measured in the range 0-10 ms following illumination by a short
laser or xenon flash, the induction kinetics reflect the following reactions:
The half-times and fluorescence yield (fF)
after a flash are shown below (t½ values and reactants
vary with starting S-state (value for n) and the initial state of QB.):
low
fF high
fF high
fF low
fF
Sn.Z.P.QA.QB ==> Sn.Z.P+.QA-.QB
==> Sn.Z+.P.QA-.QB ==>
Sn+1.Z.P.QA-.QB ==> Sn+1.Z.P.QA.QB-
<1
ns 20
- 200 ns 30
µs - 1.5 ms 150-400
µs
The fluorescence rise kinetics over the time scale < 2 µs reflect
reduction of P680+ (a quencher) (eq. 4),
Sn.Z.P+ <===> Sn.Z+.P
{4}
Sn.Z+.P <===> S(n+1)+.Z.P
{5}
A phase with t½ = ~ 30 - 400 µs reflects reduction of the
oxidized donor tyrosine (YZ+, shown as Z+
in the equations) by the S-states and the displacement of the equilibration
between states Z+.P and Z.P+ (eq. 5). In the absence
of DCMU, this phase is convoluted with the contributions from oxidation
of QA- (see below).
Longer lived low fluorescence states show inhibition on the donor side.
By exploring the flash number dependence of these effects, the site of
inhibition on the donor side can be located. Because the half-times on
acceptor and donor sides vary with flash number, the binary and quartenary
patterns associated with the two-electron gate and the OEC respectively,
can be measured by appropriate choice of time after the actinic flash,
and used to assay these reactions.
Kinetics of the two-electron gate
Kinetics in the range 30 µs - 2 ms reflect the oxidation of QA-.
The kinetics depend on the state of QB, and are more rapid when
QB is oxidized then when it is in the semiquinone state (QB-)
before the flash. When the pool quinone and QB areoxidized,
the QB-site can be either occupied or vacant, giving mixed first-order
and pseudo-first order kinetics following a flash from the dark-adapted
state. These differential kinetics can be used to explore the
binary pattern of the two-electron gate, and the kinetic and thermodynamic
parameters which describe its mechanism.
Nonphotochemical quenching of fluorescence
Plants protect themselves against excess light by switching on additional
pathways for dissipation ("exciton dumping"), through a mechanism
which leads to a lowering of fluorescence, and is general called non-photochemical
quenching of fluorescence, or qE-quenching (the subscript E
refers to the dependence on the state of "energization" of the
chloroplast membrane by the proton gradient). This dissipative pathway
requires two conditions, both of which depend on the lumenal pH:
- When lumenal pH falls below ~5.5, the enzyme violaxanthin de-epoxidase
is activated, and converts violaxanthin to antheraxanthin and zeaxanthin
in the xanthophyll cycle, mainly in the minor ligh harvesting complexes
(LHCs), CP24, CP26 and CP29.
- When antheraxanthin and zeaxanthin are present in the minor LHCs, a
quenching state is produced by low lumenal pH (<5.5) which probably
reflects a change in the interaction between chlorophylls and the xanthophylls
leading to thermal efficient dissipation through energy transfer.
A brief review of the
mechanism of qE-quenching can be found here.

©Copyright 1996,
Antony Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu
