Lecture 9
Electrochemical potential
|
Generalized proton circuit
Generalized diagram of the proton circuits
for the major energy conserving electron transport chains.
Equations for transport, chemical and electrochemical potential gradients
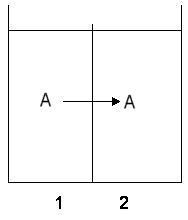
Transport of a neutral species down a chemical gradient
When a chemical species is transported between two volumes separated by
a barrier, the component undergoes a change in concentration in both compartments.
The spontaneous direction of flow will be to bring the concentrations in
the two compartments towards the same value.
Using the formalism for discussing chemical potential
we developed in a previous lecture:
m'A = moA
+ RT ln aA
we can describe the work of transport between two phases as:
DG = nDm'
Since
Dm'A(2-1) = RT ln
aA2 / aA1 -------(1)
the driving force for transport between phases 2 and 1 is negative (and
the free energy change is negative) when the flux is down the gradient
in activity (concentration gradient) across the separating barrier.
Transport of a charged species down an electrochemical chemical gradient
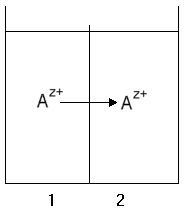 We can use a similar formalism when discussing transport of ions and metabolites
which are charged. In this case, we have to take account of the fact that
electrical work is done when charge is moved across an insulating barrier
between two phases. To do this, we have to consider that the activity of
a charged species depends on the local electrostatic potential, and the
work of transport will include the electrical work associated with movement
of charge between phases of different potential. To describe this, we need
a new term, the electrochemical potential of our charged species, defined
as the sum of the chemical and electrical potentials for the component
(see definitions of work terms in lecture
3). The effect of electrostatic potential will depend on the number of
charges, z, carried by the component, giving:
m = m'
+ zFy
Dm(2-1)
= Dm'(2-1) + zFDy(2-1)
Dm(2-1)
= RT ln aA2 / aA1 + zFDy(2-1)
-----(2)
We can use a similar formalism when discussing transport of ions and metabolites
which are charged. In this case, we have to take account of the fact that
electrical work is done when charge is moved across an insulating barrier
between two phases. To do this, we have to consider that the activity of
a charged species depends on the local electrostatic potential, and the
work of transport will include the electrical work associated with movement
of charge between phases of different potential. To describe this, we need
a new term, the electrochemical potential of our charged species, defined
as the sum of the chemical and electrical potentials for the component
(see definitions of work terms in lecture
3). The effect of electrostatic potential will depend on the number of
charges, z, carried by the component, giving:
m = m'
+ zFy
Dm(2-1)
= Dm'(2-1) + zFDy(2-1)
Dm(2-1)
= RT ln aA2 / aA1 + zFDy(2-1)
-----(2)
Either a concentration gradient or a gradient in potential will favor
transport, but the net gradient will be the sum of the chemical and electrical
work terms.
Equations for transport of protons
Transport of protons is of particular importance
in biological energy conversion, because the proton circuit couples the
free energy changes of electron transfer reactions to the phosphorylation
of ADP to ATP, through the proton gradient. In this case, eq. (2) becomes:
Dm(2-1) / F
= Dp(2-1) = Dy(2-1) - ZDpH(2-1)
where Z = 2.303RT/zF (with a value of ~60 mV at 30o C), and
Dp is the electrochemical proton gradient, often
called the proton motive force (p.m.f.), measured in volts.
(Note on symbols
The symbol normally used for electrochemical potential is 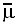 (read as mu bar). Because it is not easy to generate this symbol in HTML,
I have used m instead.)
(read as mu bar). Because it is not easy to generate this symbol in HTML,
I have used m instead.)
The equilibrium condition
A case of special interest is the equilibrium state for the transport of
an ion down a gradient.
when Dm = 0
Dy(2-1) = RT
ln aA1 / aA2
zF
and the chemical driving force is balanced by an equal and opposite electrical
force.
Click here for examples of the use of this formalism
in the description of other transport processes.
The Born equation, and permeability of lipophilic ions
Conduction across a phase is determined by the concentration of the mobile species, the conductivity of the phase for that species, the distance, and the cross-sectional area. The effectiveness of an ionophore or protonophore as an ion carrier is therefore dependent on the concentration in the membrane. The parameters determining this are given by the Born equation.
The Born energy (W) required to transfer a monovalent, spherical, nonpolarizable ion of charge e and radius a from an aqueous phase of dielectric constant e1 into the center of a bilayer of dielectric constant e2 and thickness d is:
W = e2/8pe0a {1/e1 - 1/e2} - e2/4pe0 e2d ln{2e1/(e1 + e2)}
Examination of the equation shows the terms of importance in determining the partition into the membrane. These are:
- The charge, e. Because W varies with e2, increasing the charge beyond 1 has a disproportionate effect, while decreasing the charge favors partition into the membrane. Nigericin facilitates transport by neutralizing the charge of the cation.
- The volume occupied by the charge, as represented by the radius, a. Increasing the volume allows a larger volume of local dielectric to come into play, favoring partition into the low dielectric phase. Valinomycin solvates the cation in a ring of carbonyl groups from the depsipeptide backbone, effectively increasing the volume of the charge. In protonophores like DNP or FCCP, the charge of the anion is solvated over the p-bonded system.
- The dielectric constant of the phase through which transport occurs, e2. Although this is not a variable term when dealing with a lipid membrane, insertion of protein into the lipid, or insertion of, for example, a gramicidin pore, allows transport through a phase of higher dielectric constant than that provided by the lipid.
- An additional term, not included in the Born equation, is the partition coefficient into the lipid phase. Valinomycin, nigericin and gramicidin are effective ionophores because the outside of the ionophoric complex is lipophilic. Octyl-DNP is much more effective as an uncoupler than DNP.
A large body of research has explored the ramifications of the Born equation, and shown that these factors apply, and provide a context for an understanding of ionophoric mechanism.
Physical chemistry of coupling between electron transfer and H+-transport
The physical chemistry of a model proton pump.

©Copyright 1996, Antony
Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu


![]() (read as mu bar). Because it is not easy to generate this symbol in HTML,
I have used m instead.)
(read as mu bar). Because it is not easy to generate this symbol in HTML,
I have used m instead.)