Lecture 7Redox potentiometry |
If the system under study is membrane bound, then consideration should be given to potential mediation pathways; for example, equilibration through quinone reactive sites might be best achieved through water soluble analogues of the endogenous quinones. This can be problematic, because in some cases such analogues can also act as inhibitors of the catalytic sites through which they achieve mediation.
In order to find the redox potential(s) of components in an uncharacterized system (for example, the cell membrane from a respiratory or photosynthetic bacterium which carries the electron transfer chain), the material is suspended in a stirred redox cuvette, which is placed in a spectrophotometer.
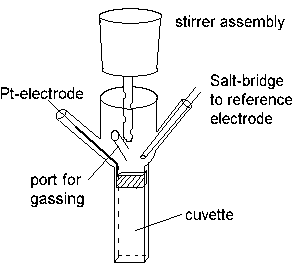
The redox potential in the cuvette is measured with an electrometer through the Pt-electrode, using an appropriate reference electrode. The system is kept anaerobic (in order to avoid electron transfer to O2) by flowing pure N2, helium, argon, or other inert gas, over the reaction mixture.
When the system has reached a stable state (no change in absorbance), a reference measurement is taken; the potential in the cuvette is then changed to a new values, and a new spectrophotometric measurement taken. The process is repeated over the redox range of interest, and a set of data obtained representing a redox titration of absorbance change as a function of ambient redox potential. Since the oxidized and reduced forms of the redox centers have different absorbance spectra, suitable choice of wavelength allows the absorbance change to be interpreted as a change in concentration of oxidized and reduced forms. The values can then be substituted into the standard equations; by repeating the experiment at different pH values, the parameters describing the redox behavior of each component (Eo', z, n, and any pK values) can be ascertained.
The simplest way to change the redox potential in the cuvette is by
addition of small aliquots of oxidant or reductant. Ferricyanide (Eo'
~ 436 mV at pH 7) and dithionite (Eo' ~ -420 mV at pH 7) are
most commonly used to cover the physiological range.
Alternatively, the potential can be change by coulometric titration,
using working electrodes to provide current to displace the redox poise
of the mediators to a new value.
The latter method lends itself better to automation, but is not without
complications.
A good test of the adequacy of a redox titration is to perform it in both oxidative and reductive directions. If the two curves coincide, it can be assumed that the reaction was carried out under the reversible conditions necessary (the electrode was in equilibrium with the mediators and the redox centers under study). If not (if the curves show "hysteresis"), the measurements under one or other (or both) titration conditions were not made at equilibrium.