Elements are defined as substances that consist of one type of atom, for example Carbon atoms make up diamond, and also graphite. Pure (24K) gold is composed of only one type of atom. Atoms are the smallest particle into which an element can be divided. The ancient Greek philosophers developed the concept of the atom, although they considered it the fundamental particle that could not be broken down. Since the work of Enrico Fermi and his colleagues, we now know that the atom is divisible, often releasing tremendous energies as in nuclear explosions or (in a controlled fashion in) thermonuclear power plants.
Subatomic particles were discovered during the 1800s. For our purposes we will concentrate only on three of them. The proton is located in the center (or nucleus) of an atom, each atom has at least one proton. Protons have a charge of +1, and a mass of approximately 1 atomic mass unit (amu). Elements differ from each other in the number of protons they have, e.g. Hydrogen has 1 proton; Helium has 2.
The neutron also is located in the atomic nucleus (except in Hydrogen). The neutron has no charge, and a mass of slightly over 1 amu. Some scientists propose the neutron is made up of a proton and electron-like particle.
The electron is a very small particle located outside the nucleus. Because they move at speeds near the speed of light the precise location of electrons is hard to pin down. Electrons occupy orbitals, or areas where they have a high statistical probability of occurring. The charge on an electron is -1. Its mass is negligible (approximately 1800 electrons are needed to equal the mass of one proton).
The atomic number is the number of protons an atom has. It is characteristic and unique for each element. The atomic mass (also referred to as the atomic weight) is the number of protons and neutrons in an atom. Atoms of an element that have differing numbers of neutrons (but a constant atomic number) are termed isotopes.
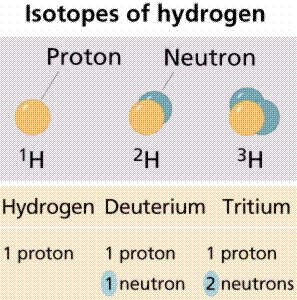
Note that each of these isotopes of hydrogen has only one proton. Isotopes differ from each other in the number of neutrons, not in the number of protons. Image from W.H. Freeman and Sinauer Associates, used by permission.
Some isotopes are radioisotopes, which spontaneously decay, releasing radioactivity. Other isotopes are stable. Examples of radioisotopes are Carbon-14 (symbol 14C), and deuterium (also known as Hydrogen-2; 2H). Stable isotopes are 12C and 1H.
The Periodic Table of the Elements provides a great deal of information about various elements. An on-line Periodic Table is available by clicking here, or if that is busy try a mirror site.
The above image is from James K. Hardy's chemistry site at the University of Akron. Each Roman numeraled column on the label (at least the ones ending in A) tells us how many electrons are in the outer shell of the atom. Each numbered row on the table tells us how many electron shells an atom has. Thus, Hydrogen, in column IA, row 1 has one electron in one shell. Phosphorous in column VA, row 3 has 5 electrons in its outer shell, and has three shells in total.
Electrons, because they move so fast (approximately at the speed of light), seem almost to straddle the fence separating energy from matter. Because of this, we think of electrons both as particles of matter (having mass is a property of matter) and as units (or quanta) of energy. When subjected to energy, electrons will acquire some of that energy.
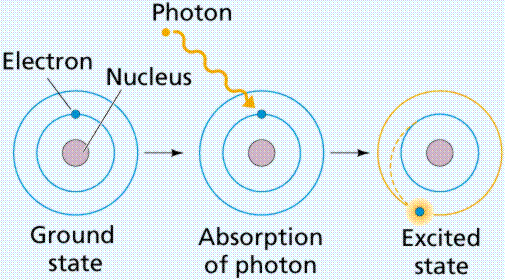
Excitation of an electron by energy, causing the electron to "jump" to another electron (energy) level known as the excited state. Image from W.H. Freeman and Sinauer Associates, used by permission.
An orbital is also an area of space in which an electron will be found 90% of the time. Orbitals, where electrons tend to occur, are of different shapes. The s orbital is spherical, and located closest to the nucleus. Since each orbital can hold a maximum of two electrons, atomic numbers above 2 must fill the other orbitals. The px, py, and pz orbitals are dumbbell shaped, along the x, y, and z axes respectively. The major energy levels (also known as shells) into which electrons fit, are (from the nucleus outward) K, L, M, and N. Sometimes these are numbered, with electron configurations being: 1s2, 2s22p1, etc. This nomenclature tells us the 1st energy level (shell) has 2 electrons in the s orbital, and 2nd energy level has 2 electrons in its s orbital, plus one electron in its p orbital.
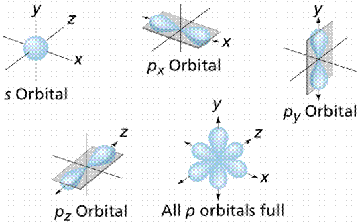
Geometry of orbitals. S-orbitals are spherical, p-orbitals are shaped like a dumbbell or figure 8. Since each orbital can hold 2 electrons max. Image from W.H. Freeman and Sinauer Associates, used by permission.
During the nineteenth century chemists arranged the then-known elements according to chemical bonding, recognizing that one group (the furthermost right column on the Periodic Table, referred to as the Inert Gases or Noble Gases) tended to occur in elemental form (in other words, not in a molecule with other elements). It was later determined that this group had outer electron shells containing two (as in the case of Helium) or eight (Neon, Xenon, Radon, Krypton, etc.) electrons.
As a general rule, for the atoms we are likely to encounter in biological systems, atoms tend to gain or lose their outer electrons to achieve a Noble Gas outer electron shell configuration. The number of electrons that are gained or lost is characteristic for each element, and ultimately determines the number and types of chemical bonds atoms of that element can form.
Ionic bonds are formed when atoms become ions by gaining or losing electrons. Chlorine is in a group of elements having seven electrons in their outer shells. Members of this group tend to gain one electron, acquiring a charge of -1. Sodium is in another group with elements having one electron in their outer shells. Members of this group tend to lose that outer electron, acquiring a charge of +1. Oppositely charged ions are attracted to each other, thus Cl- (the symbolic representation of chlorine) and Na+ (the symbol for sodium, using the Greek word natrium) form an ionic bond, becoming the molecule sodium chloride. Ionic bonds generally form between elements in Group I (having one electron in their outer shell) and Group VIIa (having seven electrons in their outer shell). Such bonds are relatively weak, and tend to disassociate in water, producing solutions that have both Na and Cl ions.
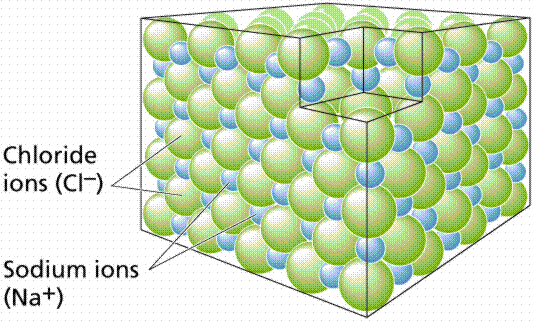
Formation of a crystal of sodium chloride. Each positively charged sodium ion is surropunded by six negatively charged chloride ions; likewise each negatively charged chloride ion is surrounded by six positively charged sodium ions. The overall effect is electrical neutrality. Image from W.H. Freeman and Sinauer Associates, used by permission.

Table Salt Crystal (SEM x625). This image is copyright Dennis Kunkel http://www.pbrc.hawaii.edu/~kunkel/gallery, used with permission.
Covalent bonds form when atoms share electrons. Remember that electrons move very fast and thus can be shared, effectively filling or emptying the outer shells of the atoms involved in the bond. Such bonds are referred to as electron-sharing bonds. An analogy can be made to child custody: the children are like electrons, and tend to spend some time with one parent and the rest of their time with their other parent. Carbon (C) is in Group IVa, meaning it has 4 electrons in its outer shell. Thus to become a "happy atom", Carbon can either gain or lose four electrons. By sharing the electrons with other atoms, Carbon can become a happy atom,. alternately filling and emptying its outer shell.
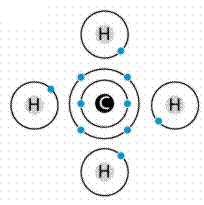
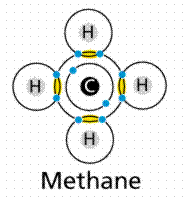
Formation of covalent bonds in methane. Carbon needs to share four electrons, in effect it has four slots. Each hydrogen provides an electron to each of these slots. At the same time each hydrogen needs to fill one slot, which is done by sharing an electron with the carbon. Image from W.H. Freeman and Sinauer Associates, used by permission.
The molecule methane (chemical formula CH4) has four covalent bonds, one between Carbon and each of the four Hydrogens. Carbon contributes an electron, and Hydrogen contributes an electron. The sharing of a single electron pair is termed a single bond. When two pairs of electrons are shared, a double bond results, as in carbon dioxide. Triple bonds are known, wherein three pairs (six electrons total) are shared as in acetylene gas or nitrogen gas.
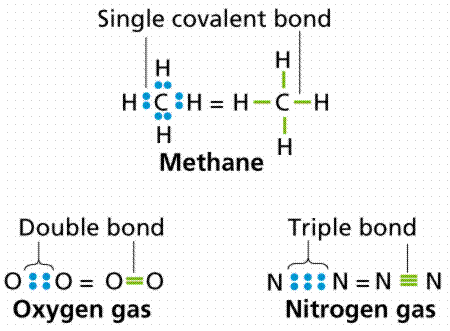
Ways of representing covalent bonds. Image from W.H. Freeman and Sinauer Associates, used by permission.
Sometimes electrons tend to spend more time with one atom than with another. In such cases a polar covalent bond develops. Water (H2O) is an example. Since the electrons spend so much time with the oxygen (oxygen having a greater electronegativity, or electron affinity) that end of the molecule acquires a slightly negative charge. Conversely, the loss of the electrons from the hydrogen end leaves a slightly positive charge. The water molecule is thus polar, having positive and negative sides.
Hydrogen bonds result from the weak electrical attraction between the positive end of one molecule and the negative end of another. Individually these bonds are very weak, although taken in a large enough quantity, the result is strong enough to hold molecules together or in a three-dimensional shape.
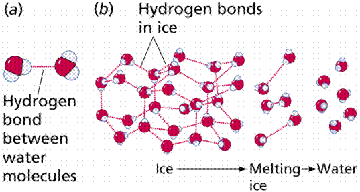
Formation of a hydrogen bond between the hydrogen side of one water molecule and the oxygen side of another water molecule. Image from W.H. Freeman and Sinauer Associates, used by permission.
Molecule versus Mixture: Molecules are compounds with elements in definite, fixed ratios. Those atoms are held together usually by one of the three bonds discussed above. For example: water, glucose, ATP. Mixtures are compounds with variable formulas/ratios of their components. For example: soil. Molecular formulas are an expression in the simplest whole-number terms of the composition of a substance. For example, the sugar glucose has 6 Carbons, 12 hydrogens, and 6 oxygens per repeating structural unit. The formula is written C6H12O6.
Chemical reactions occur in nature, and some also can be performed in a laboratory setting. Chemical Equations are linear representations of how these reactions occur. Combination reactions occur when two separate reactants are bonded together, e.g. A + B -----> AB. Disassociation reactions; occur when a compound is broken into two products, e.g. AB -----> A + B.
Biological systems, while unique to each species, are based on the chemical bonding properties of carbon. Major organic chemicals (those associated with or formed by the actions of living things) usually include some ratios of the following elements: C, H, N, O, P, S.
These learning objectives are taken from my Biology for Nonmajors class (BIO 102). I have tried to add a link to each that will direct you to a part of this chapter or another website that will facilitate your completion of the objective.
Back to Table of Contents | Continue with Chem-2
Email: mj.farabee@emcmail.maricopa.edu![]()
Last modified:
2000/01/08:15:20:28
The URL of this page is:
gened.emc.maricopa.edu/bio/BIO181/BIOBK/BioBookCHEM1.html