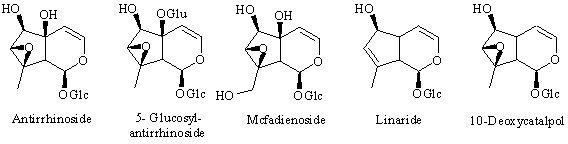
Iridoids in Antirrhinum
Antirrhinoside is one of the main constituents in Antirrhinum majus. It is an iridoid glucoside which is of terpenoid origin and it can be isolated in up to 1.5 % of wet weight in some varieties of snapdragon (e.g. "White Wonder" and & quot;Bright Eyes" [ 1] ). Iridoids are usually found in Sympetalous plant families [ 2] , but antirrhinoside is only rarely found outside Scrophulariaceae/Antirrhinae. The iridoids reported [ 3] from the genus Antirrhinum are:
Biosynthesis of antirrhinoside
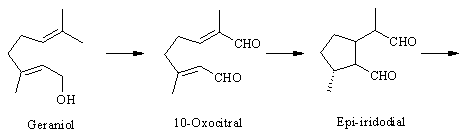
The pathway. The biosynthesis of antirrhinoside has been investigated in detail by the iridoid group (http://www.ok.dtu.dk/ok/research/srjres/srjres1.htm) at The Technical University in Lyngby, Denmark. As noted above, the iridoids are o f terpenoid origin [ 4] , and although it has not been shown experimentally, antirrhinoside like other iridoids is probably derived from geraniol. Feeding experiments have shown that epi-iridodial and epi-iridotrial are precursors giving incorporations of 0.3 and 4 % into antirr hinoside, respectively [ 5] . Note that epi-iridotrial exists in an equilibrium between the two forms shown. The next step is an oxidation of the aldehyde group to the acid, since it also has been demonstrated that epi-iridotrial glucoside is a poor precursor.
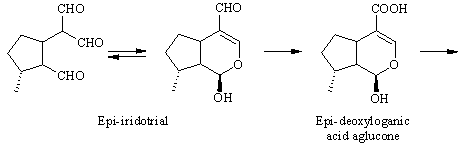
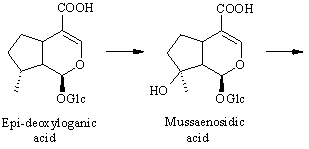
The conversion from epi-deoxyloganic acid to deoxygeniposidic acid was an enigma for some time [ 6] , but it has now been established that mussaenosidic acid is an intermediate [ 7] . The steps following deoxygeniposidic acid are straightforward, although it took many experiments to prove the exact succession of oxidative reactions. Incorporations of the late precursors are usually above 20 % [ 3,6] .
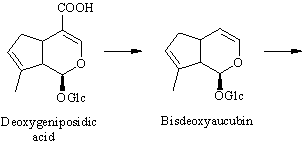
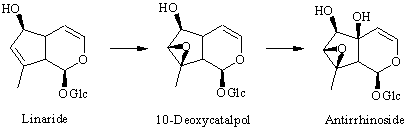
The methodology. Biosynthetic experiments can be performed in different ways. One of these is feeding with an isotopically labelled precursor in order to prove whether this is incorporated into the final product or not.
In Lyngby, we use deuterium- and/or carbon-13-labelled precursors, and the isolated product is investigated by NMR partly to measure the amount of incorporation, partly to prove the position of the label.
In a typical experiment, freshly cut stems (10 - 15 g) of actively growing plants are placed in a 10 ml beaker with water (5 ml) in which the labelled suspected precursor (10 -20 mg) has been dissolved. Starting in the morning, the plants will usually have sucked up the solution during the day and more water is added to the container. Then the plants are left for 3 to 4 days to metabolize the precursor. After isolation of the compound(s) in the plant, the incorporation is measured.
Isolation of iridoid glucosides
Iridoid glucosides are water-soluble. They are conveniently isolated by blending the fresh or frozen plant (100 g) in ethanol (400 ml), or extracting powdered dry plant material (25 g) with 80-96 % ethanol (150 ml) for 3 to 4 days. After filtering off the solid parts and evaporating the green solution, the residue is partitioned between water (10 ml) and ether (50 ml) and separated in a funnel. The aqueous extract now contains salts, sugars, and glycosides. It can now be applied to a chromatographic c olumn with reverse phase silica gel and eluted with water-methanol mixtures monitoring with a UV-detector (230 and/or 206 nm). Water will elute most salts and sugars while the iridoid (or other) glycosides will be eluted with increasing amounts of methano l. Additional separations can conveniently be carried out using prep TLC eluting with chloroform-methanol (3 : 1).
[ 1] H. Franzyk, S. M. Frederiksen and S. R. Jensen (1997). Synthesis of monoterpene piperidines from the iridoid glucoside antirrhinoside. J. Nat. Prod. 60, 1012-1016.
Abstract
: Synthesis of five novel piperidine monoterpene alkaloids using the iridoid glucoside antirrhinoside as a synthon is described. Two strategies for their preparation were investigated: the first possible pathway involved an intermediate diol from which the piperidine ring was expected to be constructed via reaction of its ditosylate with an amine; the second strategy involved a double reductive amination as the key step to the piperidine ring, which proved successful. The st ereochemistry of C-5 and C-9 in the obtained piperidine monoterpenes was the same as that reported for alpha-skytanthine, a known isolate from Skytanthus acutus (Apocynaceae).
http://pubs.acs.org/isubscribe/journals/jnprdf/jtext.cgi?jnprdf/60/i10/html/np9702648.html
[ 2] S. R. Jensen (1992). Systematic implications of the distribution of iridoids and other chemical compounds in the Loganiaceae and other families of the Asteridae. Ann. Missouri. Bot. Gard. 79, 284-302.
Abstract
: The distribution of the chemical compounds iridoids, anthraquinones, and verbascosides is demonstrated in Dahlgrenograms. An analysis of iridoid biosynthesis and structure allows distinction between two main groups of comp ounds. Thus, the biosynthetic route I gives rise to the seco-iridoids and their derivatives, and another (route II) to aucubin and similar decarboxylated iridoid glucosides. Seco-iridoids from route I are widely distributed in Cornana e, Loasanae, and Gentiananae but never in Lamianae Aucubin-like compounds derived by route II are commonly found in Lamianae and in three small families in Cornanae, but are not found in Gentiananae. Ericanae contain both groups, but not within the same order. Likewise, two biosynthetically different groups of anthraquinones can be distinguished, one of which is found solely in Gentiananae and Lamianae, and thus suggests the monophyletic origin of these taxa. The distribution of verbascosides, a gr oup of caffeic acid esters, and cornoside, a compound that is often vicarious for iridoids, is shown to be limited to Lamianae and Oleaceae (Gentiananae), barring a few exceptions. This, together with other evidence, may suggest that Oleaceae systematical ly belong close to Scrophulariacaeae, despite the presence of seco-iridoids in Oleaceae. The results of an investigation of the family Loganiaceae, as delineated recently by Leeuwenberg, are presented and analysed in the light of the above distributional patterns. The chemical data, combined with a few morphological characters, reveal that the tribes Spigelieae, Loganieae, Gelsemieae, and Antonieae show many similarities and are characterized by containing seco-iridoids (biosynthetic route I), and by having intraxylary phloem and nuclear endosperm formation, and by lacking verbascosides. The tribe Potalieae share this set of characters, but because of the presence of a unique combination of compounds, elsewhere only found in Gentianaceae, it may f it better in that family. The tribes Plocospermeae, Buddlejeae, and Retzieae, as well as the genus Polypremum from Spigelieae, does not belong in the Gentiananae, because they are different in the above set of characters. Chemically (and morphologi cally), they are more closely related to Scrophulariaceae and its allies or, alternatively, Oleaceae. Our studies have revealed nothing conclusive about the tribe Desfontainieae.
[ 3] S. Damtoft, S. R. Jensen and M. Schacht (1995). Last stages in the biosynthesis of antirrhinoside. Phytochemistry 39, 549-551.
Abstract
: Feeding experiments with 2H-labeled precursors have now shown that the last steps in the biosynthesis of antirrhinoside in Antirrhinum majus involve an initial hydroxylation of the 6-position of 6,10-dideoxyaucubin to give linaride (10-deoxyaucubin), followed by epoxidation to give 10-deoxycatalpol (5-deoxyantirrhinoside) and finally hydroxylation of the 5-positon to give antirrhinoside. 10-Deoxycatalpol was prepared by epoxidation of 10-deoxyaucubin with H2O2/WO3. Additionally, the iridoid content of two other species of Antirrhinum, namely A. speciosum and A. sicculum was investigated. In the first of these 10-deoxycatalpol was isolated for the first time from a plant together with antirrhinos ide, linaride (10-deoxyaucubin) and macfadienoside. Antirrhinum sicculum contained 5-glucocyl antirrhinoside as the main iridoid together with antirrhinoside and macfadienoside.
[ 4] S. R. Jensen (1991). Plant iridoids, their biosynthesis and distribution in angiosperms. Pp 133-158 in Proceedings of the Phytochemical Society of Europe (J. B. Harborne and F. A. Tomas-Barbaran (eds)), Ecological Chemistry and Biochemistry of Plant T erpenoids. Clarendon Press. Oxford.
[ 5] J. Breinholt, S. Damtoft, H, Demuth, S. R. Jensen and B. J. Nielsen (1992). Biosynthesis of antirrhinoside in Antirrhinum majus. Phytochemistry 31, 795-797.
Abstract
: Feeding experiments with deuterium labelled 8-epi-iridodial glucoside, 8-epi-iridotrial glucoside and the corresponding aglucones gave incorporation of all compounds into antirrhinoside in Antirrhinum majus. A higher incor poration of 8-epi-iridotrial than of 8-epi-iridotrial glucoside indicates that the former is an intermediate in the biosynthesis of antirrhinoside although a definite proof could not be obtained.
[ 6] S. Damtoft, S. R. Jensen and C. U. Jessen (1993). Intermediates between 8-epi-loganic acid and 6,10-deoxyaucubin in the biosynthesis of antirrhinoside. Phytochemistry 33, 1087-1088.
Abstract
: Deuterium-labelled samples of 8-epi-deoxyloganic acid, deoxygeniposidic acid and 6,10-dideoxyaucubin were all efficiently incorporated into antirrhinoside in Antirrhinum majus. Dilution experiments with labelled 8-epi-deox yloganic acid and unlabelled mussaenosidic acid, deoxygeniposidic acid or 6,10-dideoxyaucubin showed that 8-epi-deoxyloganic acid is hydroxylated in the 8-position to give mussaenosidic acid and that deoxygeniposidic acid and 6,10-dideoxyaucubin are inter mediates in the biosynthesis of antirrhinoside.
[ 7] S. R. Jensen and L. Ravnkilde. - Unpublished.
This page was written by
Søren Rosendal Jensen
Dept. Organic Chemistry, DTU, Lyngby, Denmark
fax +45 45933968
http://www.dtu.dk/ok/research/srjres/srjres1.htm