Polymeric isoprene derivatives are a large family of substances of little functional and structural common ground: steroids, carotenoids, gibberelic acid are just some of its members.
Several thousand different types of molecules from very different plant groups have been isolated and characterized. Despite their varied structures, all of them are synthesized by only a few pathways.
The starting product of all the different groups of compounds shown in the illustration above is mevalonic acid that is transformed into a phosphorylated isoprene upon phosphorylation. This isoprene polymerizes subsequently. In the course of polymerization, the number and position of the double bonds are fixed. All green plants are able to generate linear isoprenoids in this way.
While terpenes with more than five isoprene units are quite universal, many of the simpler terpenes are restricted to certain plant groups. Sequestiterpenes, for example, are common in mosses but occur in higher plants, too. They can be found with Magnoliales, but not with Ranunculales. This example shows, why the presence of certain secondary plant products has proven to be a useful taxonomical feature. The same is also true for monoterpenes (iridoid compounds, iridians), more about this topic can be found in the section about systematics. Among the diterpenes are the gibberellins, a group of phytohormones.
Steroids are triterpenes or triterpenoids. Triterpenes are a group of molecules that contain 30 C-atoms and are generated by the polymerization of six isoprene units although a number of derivatives, some with more but most with less C-atoms are also counted among this group. Steroid molecules consist of four rings marked A, B, C and D that have a number of additional residues R. It is of some importance whether two of these are in a cis- (i.e. at the same side of the cyclic system) or in a trans-position (at opposite sites).
Steroids have been shown to occur both in gymnosperms and in angiosperms.
Carotenoids are very common both in the plant and the animal kingdom though they are always of plant origin. All of them are tetraterpenes, i.e. they contain 40 C-atoms in eight isoprene residues. The pictures below show that they all have a centre of symmetry. They are formally derived by the subsequent hydration, dehydration, ring formation, shifting of double bonds and / or methyl groups, chain elongation or shortening and the incorporation of oxygen into a non-cyclic C40H56 compound. Carotenoids can be further classified into
![]() carotenes
(pure carbohydrates without additional groups) and the
carotenes
(pure carbohydrates without additional groups) and the
![]() xanthophylls
(carotenoids containing oxygen).
xanthophylls
(carotenoids containing oxygen).
|
beta-carotene
|
|
|
|
|
|
|
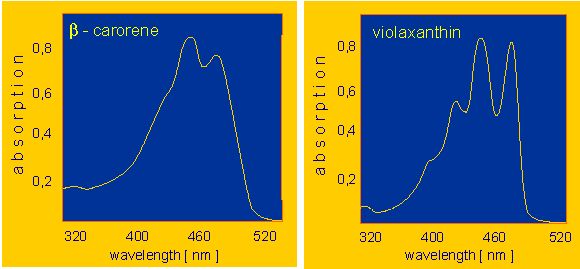
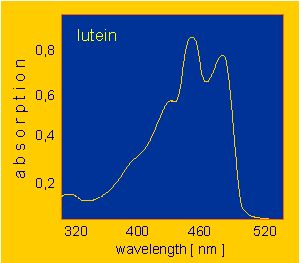
Members of both groups are components of the pigment systems (the light traps) of chloroplasts and are involved in the primary light absorption and the photon canalization of photosynthesis. Moreover, they also function as light receptors in a number of further light-induced plant processes. Some representative absorption spectra are shown below. The yellow colour of many flowers is caused by carotenoid-containing chromoplasts that are usually devoid of chlorophyll. Carotenoids are also common in fruits. The red colour of ripe tomatoes and of pepper is caused by the presence of lycopene. It is a linear molecule with 13 double bonds, 11 of which are conjugated. Many carotenes (and xanthophylls) are cyclic at their termini and loose as a consequence the terminal double bond(s).
If such a ring is formed at just one terminus, then the result is gamma-carotene, if they occur at both, either alpha- or beta - carotene is formed depending on the type of ring generated . Beta-carotene is best-known as the pigment of the carrot (Daucus carota). It occurs mostly as a crystal. One of its most important derivatives is vitamin A (a precursor of visual purple).
Xanthophylls like the common xanthophyll of green leaves or lutein are derived from carotenes. Violaxanthin, for example, is a derivative of alpha-carotene. The yellow pigment of corn, zeaxanthin is a beta-carotene derivative. Fucoxanthin, the brownish pigment of brown algae and diatoms, is another xanthophyll. Among the catabolic products of xanthophyll is the pigment of saffron, the crocetin.
|
|