Microtubules are components of all eucaryotic cells. They participate in a range of motion processes, like:
| der the movements of flagella and cilia, | |
| the movements of chromosomes during meiosis and mitosis and | |
| the transport of granules and vesicles within the cells that effects cell wall formation, shape and specialization of the cells. |
Microtubules can form regular complex structures. All flagella and cilia of eucaryotes are characterized by the so-called '9+2' structure.
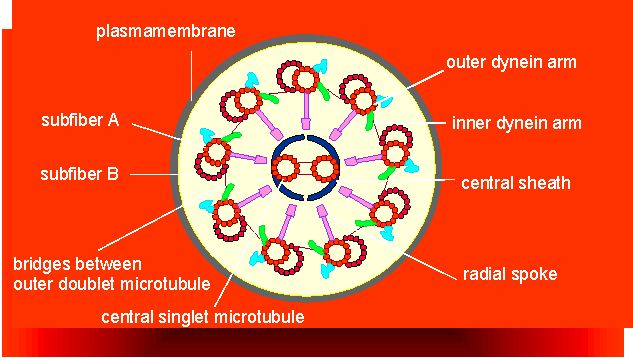
The microtubuli of plant cells in their interphase state are closely below the plasmalemma (they are cortical) and their orientation correlates strongly with that of the cell wall's cellulose fibrils. 1963 proved to be a decisive year for their research. For the first time was glutaraldehyde used by D. B. SLAUTTERBACK as well as M. C. LEDBETTER and K. R. PORTER (Rockefeller University, New York) for the fixation of electron microscopic preparations. As a consequence was the microtubuli structure stabilized and easier to describe. In the electron microscope do they look like characteristic tubules with an outer diameter of 240 Å, and an inner one of 140 Å, consequently are their walls 50 Å thick. Microtubules consist, as has been mentioned before, of tubuline subunits polymerized as helices. 13 tubuline dimers are required for one helical turn. Cilia and flagella have two tubules: a complete A and an incomplete B tubule, which differ in the lateral bindings between the tubuline dimers (alpha - beta, beta - alpha in A; alpha - alpha, beta - beta in B).
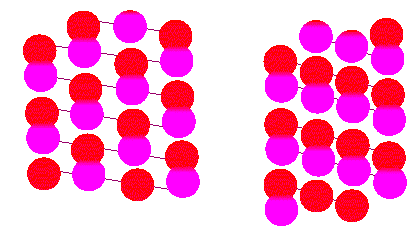
Microtubuli are tubules consisting of polymerized tubuline. Tubuline is a protein dimer out of an alpha and a beta subunit. The left tubule (surface view) is of the A type, the one to the right is type B (according to R. E. STEPHENS, K. T. EDDS, 1976).
Just as microfilaments are microtubuli, too, associated with numerous proteins. The best-known is dynein (an ATPase) required for the energy transformations of movements. Furthermore exists a whole group of proteins collectively called MAPs (microtubule associated proteins), most of which seem to have regulatory functions.
Usually exists polymerized and depolymerized tubuline in the cytoplasm in parallel, while only the polmerized form is found in the flagella. Colchicine (the alkaloid of the naked lady Colchicum autumnale), a low temperature and excess calcium ions support depolymerization. The alkaloid of the periwinkle (Vinca rosea), called vinblastine, precipitates tubuline.
It seems as if each cell would have control mechanisms at its disposal that regulate the initiation of microtubuli formation, their polymerization and depolymerization as well as their orientation within the cell. In contrast to microfilaments exist enough studies about their significance for plant cells. Antibodies against animal tubuline react with plant one, too. The close serological relation points out that they changed only little during evolution and that their significance for the cell has been established early. For reasons of simplicity is their part in flagellar and cilia movements discussed first, afterwards is their role in the changes occurring during the cell cycle explained.
Strictly speaking are the cilia and flagellar movements of eucaryotes intracellular movements; for although a flagellum (or a cilium) seems to be an appendage of the cell, is it surrounded by the plasma membrane. All dynein molecules along the whole length of the microtubule have continuously to be supplied with sufficient amounts of ATP. Both ion milieu and pH of their surrounding has to be right.
P. SATIR (1968, 1976 at that time at the University of California, Berkeley) described the process of movement as a sliding filament mechanism where the peripheral tubuline doublets slide past each other. In the course of this contacts the dynein that is always anchored to the A tubule the B tubule of the neighbouring doublet with its tips.
This mechanism explains the forward and back strokes of the cilium but not the numerous variations of cilia movements found mainly with protozoa. There exist pulling and pushing flagella, as well as tinsel-type flagella. There exist flagella that rotate around an imaginary axis and flexible flagella where the movement spreads wave-like along the axis. Many motile cells can change into forward or reverse gear or can carry out more or less strong corrections of the course.
Cilia differ from flagella only in their number per cell. They are usually quite short and cover often the whole surface of a cell. Cilia are rare in plants, an often cited example are the zoospores of Vaucheria sessilis..
With algae (except red algae) are flagellated stages common. They are often found with the spermatozoids (male germ cells) of mosses and ferns. Early during the evolution of seed plants were flagellated stages more and more driven out. Among the few still existing exceptions are the spermatozoids of Gingko biloba and the cycads.
Movements are often controlled by extern signals. Many protists are attracted by certain sources of stimulation called taxis: light (phototactic behaviour) or certain chemicals (chemotactic behaviour). Usually follows the motion a concentration or intensity gradient. If a threshold of sensation is passed, begins a reverse reaction. During the last decades was signal recognition a much-studied topic. We know, for example, that the carotenoids within the stigma of some algae (Euglena, for example) are sensitive to blue light. The chloroplast movements of the alga Mougeotia are controlled by the phytochrome system and germ cells (of algae) react to species specific sexual attractants. But how the perceived signal is converted and how signals of the same or the opposite kind are co-ordinated in a directed movement is not even basically understood (black box).
The basis of many flagella is equipped with a complexly structured basal body. M. MELKONIAN (Botanisches Institut der Universität Köln) analyzed the basal bodies of a number of algae and found group-specific patterns. He rated these structures and their variations as traits that help to understand the family relations of the single groups of algae significantly.
It is long since known that microtubuli constitute the biggest part of the mitotic spindle and that they cause the chromosomal migrations. Their whereabouts during interphase remained a mystery for long times. A lot of electron microscopic preparations fixed with glutaraldehyde were screened. And again and again did it show that microtubuli are found mainly in the peripherous plasma. In many cases corresponds their arrangement to the layering of the cellulose microfibrills of the developing primary wall (J. D. PICKETT-HEAPS and D. H. NORTHCOTE, 1966). The assumption that their orientation determines the orientation of the wall's microfibrills suggested itself. Despite several arguments against it, does it seem as if this assumption is right. But the existence of the microtubuli is neither cause nor prerequisite of cell wall formation: cells treated with colchicine develop cell walls. Interestingly are the microfibrills of these walls arranged statistically and not well-ordered. That is why it was at first assumed that the microtubuli participate only in the transport of material required for cellulose synthesis. Another possible assumption would be that they arrange the cellulose synthase molecules within the plasma membrane and that the specific cellulose fibril pattern is a consequence of this (B. A. PALEVITZ and P. K. HEPLER, 1976). Observations of several algae and of root hairs point at such a connection. Microtubuli within the plasma can be in contact with each other, with the plasmalemma and/ or with the nuclear envelope.
In plasma tubules take microtubuli part in the shifting of secretory vesicles, organelles and other cellular particles. The tight network that surrounds the migrating generative nucleus is especially impressive. The tip of the pollen tube (the apex) contains no microtubuli but an increased number of microfilaments.
A prerequisite of the division of isolated protoplasts is the formation of a cell wall. It is preceded by the reorganization of the peripherous system of microtubuli in which the arrangement of the microtubuli determines the shape of the developing cell. While the properties described so far are of a general nature, have microtubuli of specialized cells additional functions. They participate in the control of cell wall thickening and in the lignin deposition of secondary cell walls. Treatment with colchicine prevents the development of the otherwise usual strip-shaped secondary thickenings instead develops - analogous to the effects on primary cell wall formation - an unorganized pattern of thickening elements (P. K. HEPLER and E. H. NEWCOMB, 1964, J. CRONSHAW, 1967).
M. M. FALCONER and R. W. SEAGULL (1985) analyzed the development of tracheids. Studies performed with the fluorescence microscope showed clearly that microtubuli occur wherever tracheary cell wall thickenings form. The pattern of these wall thickenings corresponds to the pattern of the microtubuli.
Differing wall thicknesses and changes of the cell's shape like those found in the development of stomata could be caused by the uneven distribution of intracellular microtubuli during morphogenesis. They are more frequent at sides with thickened walls (the periclinal walls) than at those with thin walls (the anticlinal walls).
The number of microtubuli correlates with an accumulation of dictyosomes so that it seems as if they were actually required for the lining up of material transport and the distribution of cell wall material within the cell (B. A. PALEVITZ and P. K. HEPLER, 1976, B. GALATIS, 1980). In some algae (Hydrodictyon, Pediastrum and Staurastrum, for example) do they influence the cell's shape and colony development (H. J. MARCHANT and J. PICKETT-HEAPS, 1974, H. J. MARCHANT, 1979). And in Micrasterias do they take part in the postmitotic migration of the nucleus (O. KIERMEYER, 1972), though they have no influence on the cell's shape. In contrast is the arrangement of the microtubuli in the flagellate Ochromonas that has no cell wall decisive for the development of the specific cell shape; a corresponding order can be seen in the pellicle of Euglena, where the microtubuli participate in the constant change of the cells' shapes ('metabolic movements').
Since both microtubuli and microfilaments partake in intracellular plasma movements, the migration of organelles and other particles arises the question which of the two systems is primarily responsible for a certain movement. The answer is species specific. D. MENZEL (Universität Heidelberg, 1987) discovered that the movements of organelles of the genus Caulerpa are caused by microtubuli, those of Acetabularia by microfilaments and that both systems are equally important in Bryopsis.
 What happens in a plant cell when the preparations for mitosis
(or meiosis) start? An especially striking feature of such cells
is the development of a preprophase band surrounding the nucleus.
In its plane will later on develop the phragmoplast. The preprophase
band forms by the shift of nearly all the cell's tubuline to this
region, i.e. the tubular interphase cytosceleton is dismantled
and a new one is built (P. K. HEPLER and D. H. NORTHCOTE, 1967).
Some cells like the much-studied endosperm cells of Haemanthus
katherinae lack the preprophase band. The question whether
it is necessary in order to move the nucleus into the right (central)
position, or whether the two processes are independent, was disputed
for a long time. Though it has not been settled yet, is the assumption
that the two processes are independent of each other nowadays
favoured. It could also be that the position of the band determines
the orientation of the mitotic spindle. Its formation starts with
the prophase. It is commonly agreed that the spindle consists
of microtubuli though the question how it causes the chromosome
movements is by far more difficult. Two types of microtubuli exist:
those that start at the poles and may stretch far beyond the equatorial
plane are called polar microtubuli. They overlap so that it looks
as if they would extend from pole to pole. Other microtubuli are
attached to the chromosomes' kinetochores (= centromeres) and
extend next to the poles or are associated with polar microtubuli.
They are called chromosomal or kinetochore-microtubuli.
What happens in a plant cell when the preparations for mitosis
(or meiosis) start? An especially striking feature of such cells
is the development of a preprophase band surrounding the nucleus.
In its plane will later on develop the phragmoplast. The preprophase
band forms by the shift of nearly all the cell's tubuline to this
region, i.e. the tubular interphase cytosceleton is dismantled
and a new one is built (P. K. HEPLER and D. H. NORTHCOTE, 1967).
Some cells like the much-studied endosperm cells of Haemanthus
katherinae lack the preprophase band. The question whether
it is necessary in order to move the nucleus into the right (central)
position, or whether the two processes are independent, was disputed
for a long time. Though it has not been settled yet, is the assumption
that the two processes are independent of each other nowadays
favoured. It could also be that the position of the band determines
the orientation of the mitotic spindle. Its formation starts with
the prophase. It is commonly agreed that the spindle consists
of microtubuli though the question how it causes the chromosome
movements is by far more difficult. Two types of microtubuli exist:
those that start at the poles and may stretch far beyond the equatorial
plane are called polar microtubuli. They overlap so that it looks
as if they would extend from pole to pole. Other microtubuli are
attached to the chromosomes' kinetochores (= centromeres) and
extend next to the poles or are associated with polar microtubuli.
They are called chromosomal or kinetochore-microtubuli.
Polar microtubuli initiate polymerization at the poles, while chromosomal ones start at the kinetochores.
In animal cells can centrioles be found next to the cell poles. Condensation begins in their vicinity. In most and especially in higher plants are they missing. They occur in some algae and appear in the meiosis but not the mitosis of the Charophyceae. At least in the development of plant polar microtubuli are they therefore dispensable. It is not known if they are replaced by some other structure
Numerous cytological results show that only kinetochore-attached chromosomes can be moved. This means that chromosomes must have a binding site specific for microtubuli (in chromosomes with 'diffuse' kinetochores may it include the whole surface of the chromosome). The migration towards the poles is based on an interaction of polar and kinetochore-microtubuli. In all probability are the chromosomes pulled and not pushed. The movements seems not to be based on a simple sliding mechanism. Instead is the chromosomal movement during anaphase caused by a shortening of the kinetochore-microtubuli with simultaneous elongation of the polar microtubuli (McINTOSH and KOONCE, 1989).
The question of energy transduction remains to be settled. During telophase is the spindle dismantled and the microtubuli gather in the phragmoplast area where the de novo organization of the interphase cytosceleton begins. Microtubuli and microfilaments are in close vicinity towards each other in the phragmoplast area. Whether the two systems co-operate cannot be answered yet either.
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de