What causes the extreme specificity of the host-parasite relation? It is useful to subdivide this question into a number of more detailed questions and to deal with each aspect separately in order to answer it. The questions are:
![]() How does the fungus reach the inner plant tissue?
How does the fungus reach the inner plant tissue?
![]() How does it surmount the cell wall?
How does it surmount the cell wall?
![]() What kind of influence have toxins on the plasma membrane?
What kind of influence have toxins on the plasma membrane?
![]() How do plant cells react to the activities of the fungus?
How do plant cells react to the activities of the fungus?
In the simplest case, germinating spores or hyphae penetrate the plant tissue through wounds of the epidermatic tissue, wounds of the cuticle or open stomata. Some specialists like Fusicoccum amygdali that belongs like most of the following examples to the Fungi imperfecti secrete a terpenoid (fusicoccin) that increases the influx of potassium into the cells of the stomata and induces thereby a permanent opening of the stomata. The consequent high loss of water causes finally the perishing of the plant. Fusicoccin is therefore also known as a wilting toxin.
The toxins – in this case cyclic peptides – of Helminthosporium maydis have an exactly reversed effect. H. maydis is the causative agent of a corn disease that effects mainly corn of the genotype Texas male sterility cytoplasm – CMS. It brought about one of the worst epidemics ever caused by fungi in the United States in 1970. Its toxins inhibit the light-induced potassium-uptake through the stomata. This lowers transpiration and consequently also the activity of photosynthesis (C. J. ARNTZEN et al., 1973).
The methods for the clarification of this topic were developed in the research group of P. ALBERSHEIM (Complex Carbohydrate Research Center, Athens, Georgia). The experimental system consisted of a fungus (usually Colletrotrichum lindemuthianum) and the host plant Phaseolus vulgaris.
The cell-wall-degrading enzymes secreted by the fungus were studied with isolated walls of the host plant’s hypocotyl as the substratum. ALBERSHEIM et al. found polygalacturonases and related enzymes, especially alpha-galactosidase, beta-galactosidase, beta-xylosidase, and alpha-arabinosidase. These enzymes were able to degrade walls of small five-day-old hypocotyl pieces completely, while pieces of 18-day-old hypocotyl largely resisted the enzymatic attack. 18-day-old seedlings have already developed secondary cell walls and their degradation requires other enzymes.
It may strike you that the mentioned enzymes attack only the pectin and cellulose fraction of the cell wall, but not its cellulose scaffolding. The fragments produced by the partial enzymatic degradation are called oligosaccharines. They do play an important part in the protection against fungi, too, as we will explain later. The basic structure of the cell remains rather intact. It is only locally perforated so that the cells stay alive. Numerous saprophytic fungi produce large amounts of cellulase and cover their own need for carbon by the degradation of cellulose.
The synthesis of alpha-galactosidase by Colletrotrichum can be induced. It takes only place in the presence of cell walls from non-resistant hosts. It seems that an extern signal generated by the host cell is required. The cell walls contain on the other hand also specific inhibitors of alpha-galactosidase. The inhibitor isolated from Phaseolus vulgaris inactivates the enzyme from Colletrotrichum forty times more effective than the respective enzyme from Fusarium oxysporum (a parasite of tomato). It has no effect on the respective enzyme from Sclerotium rolfsii. The inhibitor is a glycoprotein with the characteristics of lectin, i.e. with an affinity for certain sugar residues. This again means that the polygalactosidases from the different fungi are glycoproteins, too, and that their glycosylation pattern is obviously important for the specificity of the host-parasite interactions.
The enzyme pattern of a number of meanwhile tested fungus species differs quantitatively and qualitatively from Colletrotrichum. Fusarium oxysporum, for example, produces an enzyme that degrades polygalacturonic acid as soon as it has infected tomatoes. Rhizostonia solani produces at first the same set of enzymes as Colletrotrichum, and in a subsequent phase phenoloxydases that enable the fungus to attack secondary walls.
Phytophora infestans secretes two polygalacturonases, four galactanases, and two pectinesterases. The respective enzymes differ in their substrate specificity and in their rate of turnover. The polygalacturonases release less than six percent of the carbohydrates of potato cell walls, the galactanases release 23 percent (M. C. JARVIS et al., 1981).
G. STROBEL et al. (Montana State University, Bozeman) looked into Helminthosporium sacchari, the causative agent of the eyespot disease of sugar cane. STROBEL was not that much interested in the question why a plant is resistant against a fungus (which is the rule), but took more interest in why it is susceptive. Fungus-infected leaves of sugar cane contain the mycelium as small, stigmata-shaped centres of infection (lesions), from where several centimetre-long, red-brownish stripes spread in parallel to the leaf axis. These stripes contain no mycelium, so that it suggested itself to search for a small, diffusible toxin molecule as the cause of the symptoms. Such a substance was isolated from infected leaves and was at first identified as 2-hydroxycyclopropyl-alpha-D-galactopyranosid (helminthosporosid) with the help of nuclear resonance analysis and mass spectroscopy. Closer analysis showed that it was a mixture of three isomer sesquiterpen-alkaloids, that contain digalactosyl residues at both ends of the molecule, though. They are in the usually rare furanose state.
Helminthosporosid does not occur in healthy leaves. If leaves of sugar cane are sprayed with a solution of this toxin, then the fungus-specific symptoms appear. This fact animated G. W. STEINER (Hawaiian Sugar Planters Association) to develop a fast method for the selection of resistant sugar cane seedlings. It is used routinely in the Hawaiian cultivation programs. Helminthosporid resembles alpha-galactosides like the disaccharide melebiose and the trisaccharide raffinose. Both sugars occur in plant tissues and it is known that plant cells take them up actively.
At the plasma membrane, the toxin attaches to the same binding site as these sugars, but it is, in contrast to them, not transported into the cell. Cells do not depend on alpha-galactosides and consequently is the binding of the toxin not the sole cause for its toxicity. The alpha-galactoside-binding protein is a tetramer consisting of four identical polypeptide chains of a molecular weight of 12,000 (= about 110 amino acids). It occurs in resistant sugar cane phyli, too, though it does neither bind the sugars nor the toxin. It can be made active by treating it with a mild detergent. Analyses of its amino acid composition showed that - dependent on the respective phylum - one to four of the 110 amino acid residues are changed. This changes the whole conformation of the molecule that looses as a result its substrate affinity. It seems that this receptor protein is in the plasma membrane in close vicinity to a potassium/ magnesium pump, since its binding to the toxin increases the activity of the pump (allosteric effect). The uptake of potassium rises. The consequences are an increased uptake of water, a bigger osmotic pressure, damage of the membrane, and finally the disintegration of the cell.
Plant cells produce a wide range of secondary plant substances, and many of them prevent the growth of fungi. In addition, they produce specific phytoalexines as a reaction to an infection (K. O. MÜLLER and H. BÖRGER, 1941).
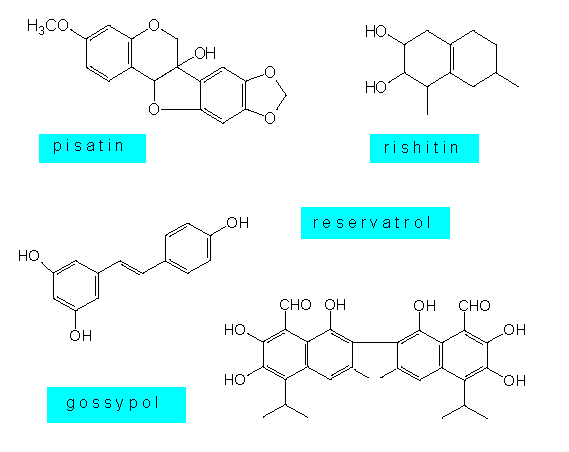
Phytoalexines are lipophilic substances, whose chemical structure poses them among the secondary plant substances. The phytoalexines of the Solanaceae and the Malvaceae are usually sequestiterpenes, that of the Leguminosae are isoflavonoids or polyacetylenes, and the phytoalexines of orchids are dihydrophenanthrenes. Some plant species like the potato produce several similar substances at the same time. Little is known about their mode of action. Some results point at an effect that changes the membrane properties of the fungus cell. Other substances seem to block the oxidative phosphorylation, and still others can link up DNA molecules. Phytoalexines provide no absolute protection against fungus infections. They are mainly directed at ‘non-pathogenic’ species. Many parasites are able to protect themselves against these substances or to develop defence mechanisms of their own.
How is the production of phytoalexines induced? P. ALBERSHEIMER et al. were able to induce a production of phytoalexines in cultures of soy bean cells as a reaction towards externally supplied polysaccharides and oligosaccharides at the beginning of the seventies. A beta-1,3-glucan with lateral branchings (beta-3,6) and a molecular weight around 10,000 proved to be especially effective.
Besides chitin, such polysaccharides are a main structural component of the walls of many fungi (ascomycetes and basidiomycetes). This means, that a component of the fungus cell wall activates the defence reaction of the plant. At low fungus growth, the phytoalexines can accumulate and reach concentrations that are toxic for the fungus. If, nevertheless, the fungus belongs to a fast-growing species, then it can spread (and damage the plant) before the defence effect of the phytoalexines come to fruition. Specific eliciting substances are called elicitors (lat.: elegere = to choose). The plant cell wall has to contain specific molecules that recognize the elicitors, since they belong to the polysaccharides. They are per definitionem lectins and it looks, as if the recognition of parasites would be among their main functions. The lectin of wheat seedlings (WGA) has a high affinity for chitin, and it is known that it stops the spreading of fungus hyphae.
One link of the causal chain of cause and effect is still missing. Until now, it is not known how the signal (the binding of a polysaccharide to the outside of the cell wall) is conducted to the inside of the cell wall and from there through the plasmalemma into the cell lumen.
Hypersensitivity: Many plant species, or ‘resistant’ phyli, respectively, react towards the induction by fungi, viruses, and other causative agents, or towards mechanical damage with hypersensitivity. It is characterized by the withering of a local section of tissue. It cuts the parasite off the nutrient supply, its opportunity to spread is taken away, and often are phenolic compounds set free, that may also lead to its death. The dead, often brownish spots on the plant are called necroses.
 Hypersensitivity is no general reaction. Only certain plant species react towards
certain parasites in this way. Genetic analyses showed, that both host and parasite
have to have dominant alleles of certain ‘resistance genes’. It is therefore also talked
of a gene-to-gene interaction. The resistance-mediating genes are termed R, the genes of
the parasite that cause the avirulence, i.e. that do not cause disease symptoms are called
A. The combination R-A forms a stop-signal. Host and parasite are incompatible. This
seemingly simple relation shows that the recognition can be analyzed genetically.
The probability to obtain resistant forms of (all?) cultured plants is therefore
pretty large. This possibility is qualified by the fact that a whole number of
independent R-loci exist. On the other hand develop new and possibly infective
fungus types by mutation that could spread on formerly resistant plants or on plants
that were especially cultivated for resistance.
Hypersensitivity is no general reaction. Only certain plant species react towards
certain parasites in this way. Genetic analyses showed, that both host and parasite
have to have dominant alleles of certain ‘resistance genes’. It is therefore also talked
of a gene-to-gene interaction. The resistance-mediating genes are termed R, the genes of
the parasite that cause the avirulence, i.e. that do not cause disease symptoms are called
A. The combination R-A forms a stop-signal. Host and parasite are incompatible. This
seemingly simple relation shows that the recognition can be analyzed genetically.
The probability to obtain resistant forms of (all?) cultured plants is therefore
pretty large. This possibility is qualified by the fact that a whole number of
independent R-loci exist. On the other hand develop new and possibly infective
fungus types by mutation that could spread on formerly resistant plants or on plants
that were especially cultivated for resistance.
Today, it is tried, too, to obtain resistance against fungi with the help of gene technology. The logistic and the proceeding of such an experiment are described in: W. SCHUCHERT: "Die Pilz-resistente Kartoffel. Gentechnisch induzierter Schutz vor der Kraut- und Knollenfäule" (German version only)
|
|