The elements required for living systems are mostly stable. Radioisotopes play a minor role, and even the half-value period of 5770 years of 14C is much higher than the life of the average organism. As a consequence, all elements of a biosphere are preserved. All that changes is their distribution within the biosphere, some are taken to new places, others are chemically integrated into different molecular components.
All changes can be described by cycles. Cycles can be drawn up for any element, but in biology, that of oxygen, carbon, nitrogen, phosphor, sulphur, as well as water are of interest. Water is no element, but a largely stable molecule that is essential for all processes of life. Cycles can be described as systems just like the generalized ecosystem that we just got to know. Often, large pools of elements like the earth's crust, the ocean or the atmosphere are the system elements. Only a minute part of the material mills around and even less participates in the development of living systems.
 The water cycle is the first to be introduced as the water supply of the
earth's surface is besides the sun and the amount of energy provided by it
the most important precondition of vegetation and thus also of the colonization
of the earth. This known fact becomes especially impressive through satellite
takings made of partially irrigated dry areas.
The water cycle is the first to be introduced as the water supply of the
earth's surface is besides the sun and the amount of energy provided by it
the most important precondition of vegetation and thus also of the colonization
of the earth. This known fact becomes especially impressive through satellite
takings made of partially irrigated dry areas.
Water can exist in three different states: solid (ice), fluid, and gaseous (vapor). It is in its fluid state at temperatures that are optimal for physiological processes. Water has a high heat storage capacity. It is moreover an ideal solvent for numerous ions. The pH-value of underground water fluctuates between pH 3 and pH 10. It is high in the coastal areas of the oceans and still higher in the open ocean. The high alkalinity is one of the main courses of the water's high capacity for carbon dioxide that becomes carbonate/bicarbonate in the water. Only a fraction of the existing water is altered chemically. The photolysis taking part during photosynthesis makes up the main part of this fraction.
The earth surface is 510 million square kilometers, 362 million of it are covered by water and 325 million square kilometers of it are open oceans. The total amount of water is 1.5 billion square kilometers. 97 percent of it is ocean water. Only three percent is freshwater, and three quarters of it is immobile ice that makes up polar icecaps and glaciers. Its atmospheric part is lower than 0.001 percent. The amount that is available for plants has roughly the same magnitude.
 Atmospheric water has a fluctuating geographic distribution. It occurs mainly
in the equator region. Water remains an average 9-10 days in the atmosphere,
though it can return to earth after a few hours or after weeks. The atmospheric
differences in temperature and pressure are the main cause of air circulation.
Above the oceans, less water comes down than enters the atmosphere
(107-114 centimeter (cm)/year as compared to 116 - 124 cm/year).
The situation is just the opposite above land. Here, 47 cm/year evaporate
into the atmosphere, while 71 cm/year precipitate. The difference is balanced
out by the draining away of surface water, especially rivers, and to a lesser
extend by underground water (seepage water). Two further aspects are important
for a botanist: the difference in the geographic distribution of the rainfall
(see also the chapter about vegetational belts )
and the part of the cycle that the plant itself takes part in.
Atmospheric water has a fluctuating geographic distribution. It occurs mainly
in the equator region. Water remains an average 9-10 days in the atmosphere,
though it can return to earth after a few hours or after weeks. The atmospheric
differences in temperature and pressure are the main cause of air circulation.
Above the oceans, less water comes down than enters the atmosphere
(107-114 centimeter (cm)/year as compared to 116 - 124 cm/year).
The situation is just the opposite above land. Here, 47 cm/year evaporate
into the atmosphere, while 71 cm/year precipitate. The difference is balanced
out by the draining away of surface water, especially rivers, and to a lesser
extend by underground water (seepage water). Two further aspects are important
for a botanist: the difference in the geographic distribution of the rainfall
(see also the chapter about vegetational belts )
and the part of the cycle that the plant itself takes part in.
The production of 20 tons of biomass requires 2,000 tons of water. The largest part of the water does thus pass the plant leaving it by way of transpiration. 15 of the 20 tons of fresh weight are made up by unbound water within the plant tissue. The other five are dry weight. Three of them are bound water, the rest consist of other substances.
The oxygen of the atmosphere is almost completely the product of the photosynthetic activity of green plants. The oxygen content of the atmosphere increased continuously during earth history since the first occurrence of organisms that were able to split water and perform photosynthesis. The increase stopped when a balance, today's 21 percentage by volume was reached. The source of the atmospheric oxygen was mainly water and to a lesser extend other oxides. Respiration and combustion are energy-consuming processes. The end product of respiration is carbon dioxide. The redox reactions taking place within organisms are discussed here.
The atmosphere contains about 1.3 x 1014 tons of free oxygen. The lithosphere carries even 5.5 x 1016 tons of bound oxygen, more than a hundred times more than the atmosphere. Its main part is bound as carbonates, silicates, sulfates, and other oxides. In the atmosphere, oxygen occurs mainly unbound, while the strongly ionizing cosmic radiation causes the production of ozone and atomic oxygen within the stratosphere. The ozone layer protects the biosphere effectively from short-wave UV-radiation.
Over the decades, human activities have used up increasing amounts of oxygen while carbon dioxide is set free. A decrease of free oxygen is still very unlikely. A noticeable increase in the atmosphere's concentration of carbon dioxide can nevertheless be registered.
A main characteristic of the oxygen cycle is its connection with a part of the carbon cycle that plants have a key position in. The exchange rate of atmospheric oxygen is a relatively high: 2,000 years. In other words: plants produce 1/2000 of the whole atmospheric oxygen each year and the same amount of oxygen is consumed by oxidation. The carbon dioxide of the atmosphere is completely exchanged within 300 years. The total amount of water, namely the already mentioned 1.5 billion cubic kilometer is completely split by photolysis and reproduced by oxidation within 2 million years. These values show the water cycle changes just little without the activity of plants, while the oxygen and the carbonate cycle would be altered drastically without plants.
Carbon is an element that occurs for the smallest part as atoms at the earth surface (carbon, diamonds). Its main part is either oxidized (carbon dioxide, carbonate/bicarbonate and a small amount of carbon monoxide) or reduced (hydrocarbons and their derivatives). The main part of the carbon of organic substances is reduced, and the fixation of carbon occurs during photosynthesis. The plants have thus a key role again. The carbon cycle is directly linked to their energy budget.
Often, a subsection of the carbon cycle, the carbonate cycle, is excluded and discussed separately. This may be practical as the turnover of carbon dioxide and water-dissolved carbonate/ bicarbonate is especially easy to determine. The 14C-method helped essentially to understand the whereabouts of the carbon.
In the carbonate cycle, the reduced state of carbon is usually treated as a 'black box'. We do indeed know least about it. It contains the living substance (biomass) but also dead material that is often collectively called fossil fuel. The amount of the carbon fixed by plants is directly proportional to the gross primary production, while the amount of carbon dioxide that is set free by respiration depends on the difference between gross and net production.
The main part of carbon dioxide exists as carbonate in the lithosphere. A smaller part is dissolved in the oceans. Carbon dioxide comes to 0.03 volume percent, i.e. 300 parts per million (ppm) of the atmosphere. This is a guideline. The carbon dioxide concentration increases continuously since the late 1950th. The function given in the following illustration can be extrapolated backwards showing that the carbon dioxide concentration must have been 260 ppm in pre-industrial times. The carbon dioxide content of the air increased about 8 percent between 1958 and 1982. This increase was caused by the burning of fossil fuels like coal, oil, and natural gas.
Based on details from 1979 and 1980, the amount of carbon dioxide can be calculated up to 5.3 billion tons per year. A further 1,8 - 4.7 x 109 tons of carbon set free as the result of the destruction of the biosphere have to be added. Among this destruction is the clearing of the tropics, the destruction of the savannas as well as frequent plowing of cultivated areas. Breaking up the soil causes carbon dioxide that was produced as the result of the decomposition of organic material to surface, its absorption to humus particles does not take place, the carbon dioxide escapes into the atmosphere or is washed away into rivers and finally into the sea. The numbers given for the atmospheric carbon dioxide concentration are average values. The concentrations may fluctuate considerably regionally and locally as a measurements close to a patch of forest show. Besides day-night fluctuations different values were captured at different heights above the soil.
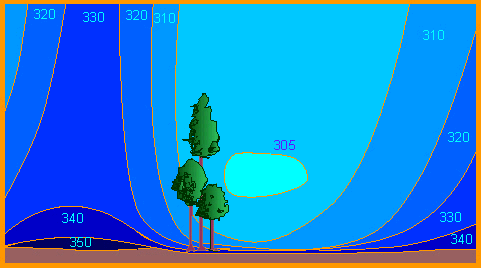
The lowest concentration, 305 parts per million (ppm) is given in turquoise, the highest concentration, 350 ppm, is given in dark blue. The vertical distribution of carbon dioxide in the air around a group of trees fluctuates with the time of the day. Photosynthesis is shut off at night and as a consequence the respiration from the soil can raise the concentration of carbon dioxide at ground level to as much as 400 ppm, while the CO2 concentration at treetop level can drop to 305 ppm at noon owing to photosynthetic uptake (A. BAUMGARTNER, 1968, according to R. MILLER and J. RUSCH, 1960).
Some quantitative data have to be considered in order to understand the global shifts of carbon. Its total amount is 1.384 x 1018 tons, 3.9 x 1013 of which are inorganic and 1 x 1012 tons are organic. The total biomass contains 5.6 x 1012 tons of carbon, its annual gross primary production covers 1.1 - 1.2 x 1011 tons of carbon, the net primary production is 0.57 x 1011 tons of carbon. About half of the gross primary production, 0.43 x 1011 tons of carbon exactly, is made up by marine plants, usually single-celled algae. In other words: the main part of the carbon fixed by primary production is respired either directly or during the biological decomposition of dead organic material.
The lifetime of marine organisms lasts weeks, that of terrestrials years. The expanded lifetime and the associated accumulation of fixed carbon results in a decreased respiratory activity. Only 30 to 40 percent are respired. A model of the global carbon cycle is given in the next illustration.
The given numbers and especially the increase in atmospheric carbon dioxide and the high percentage of the biosphere's destruction lead to the assumption that the carbon cycle is not in balance any more and that this results inevitably in an increased temperature (greenhouse effect). The reality is far more complex since only a small fraction of the carbon dioxide that is set free during combustion enters the atmosphere and remains there. A considerable part is absorbed by the oceans. There it is used for the building up of the organisms' skeletons. The importance of this process is demonstrated by the existence of extensive chalk layers and the fact that an enhanced chalk formation has been the immediate cause for the name of a whole geological era.
Hardly any hints that the annual production of biomass decreases exist despite the destruction of the vegetation by man in our time. The contrary seems to be the case, maybe because carbon dioxide has always been a limiting factor of photosynthesis. This facts should nevertheless not be overly interpreted as too high concentrations of carbon dioxide cause severe growth disorders as lab experiments showed.
The carbon turnover of terrestrial organisms can only be understood if lithosphere, surface water, atmosphere, and oceans are included. The oceans nevertheless proved to be almost closed partial cycles.
The amount of carbon exchange between ocean and atmosphere are very small. Despite the large mass shifting of the surface water more commonly known as currents the water of the deep sea is hardly affected. Measurements of its 14C-content showed that below 1,500 meters depth the water remains in the Atlantic for 275 years in the same place.
The nitrogen cycle is far more complex than the cycles you got to know until now. 79 percent of the atmosphere consist of free nitrogen and at least the same amount of bound nitrogen is found in the lithosphere. These large reservoirs are not immediately available for plants. In this context, microorganisms have a central role. Nitrogen fixation is the cue. This process has already been discussed and you may remember that it is extraordinarily energy-consuming. Plants use nitrogen almost only as ammonium and nitrate ions. In organic materials, nitrogen is mainly required for the production of the amino-groups found in proteins or nucleic acids. Nitrate and nitrite bacteria convert the amino groups back to nitrate or nitrite. Nitrate-reducing bacteria living in the soil or in water reduce oxidized nitrogen compounds and do thus close the cycle. Nitrogen fixation and reduction do roughly balance one each other.
The production of ammonium compounds and nitrates is a limiting factor of plant growth. It is true that the lithosphere contains almost unlimited amounts of nitrates, but they occur mainly in depths that are unreachable for plant roots. For humans, too, it is uneconomic to exploit this pool.
Nitrogen compounds are usually very water soluble, large amounts are therefore lost by leaching. They may, especially if occurring in addition with extensive fertilizing, accumulate in lakes or ponds and cause eutrophication. You will find more about this topic subsequent to the section about the phosphorus cycle.
Many nitrogen fixing bacteria and blue-green algae are free-living, others live in symbiosis with plants like with the leguminosae Cycas or Ginko. The symbiotic species bind about ten times as much nitrogen as the free-living. Free-living bacteria and algae fix on average 1g/square meter/year. The highest measured value is 20 g/square meter/year.
The relatively high rice yields of South and South-East Asia are partly based on the occurrence of extensive populations of blue-green algae like Nostoc. They live in the shallow stagnant waters where rice cultures are cultivated. In the whole contemplation of the nitrogen cycle, global or regional changes are of secondary importance. Far more important are the local concentrations and there again mainly the concentrations in the rhizosphere of the plants.
The nitrogen balance of an ecosystem that is basically self-contained with respect to its nitrogen, an unpastured grassland, is given in the following table. Analyses of this type have repeatedly been made. Without the results gained from them, modern agriculture and modern forestry would not be possible.
| input, output, distribution | |||
| inaccessible | |||
| organic | |||
| available mineral | |||
| roots | |||
| plant sprouts | |||
| herbivores | |||
| natural fertilization | |||
| loss | |||
| artificial fertilizer |
|
||
| nitrogen fixation | |||
| animals (loss by migration) |
|||
| |
|||
Atmospheric nitrogen is mainly inaccessible while nitrogen compounds are often very reactive and toxic. An over-fertilization does therefore often lead to degeneration phenomenons and lower yields instead to a better growth. Nitrose gases are extremely poisonous and thus a main factor in acidic rain.
Of these two elements plants can only use certain groups of compounds: phosphates and sulfates. In the earth's biosphere exist no gaseous and also no reducing phosphor compounds. Gaseous sulfur compounds like hydrogen sulfide or sulfur dioxide are rare and if they occur, they cause damage.
It is not quite right to talk about a phosphorus and a sulfur cycle since the flow of material is almost exclusively linear at least when regarded in dimensions of hundreds or thousands of years. Only when taking human influences like fertilization into consideration, a cycle begins to evolve.
The phosphorus reservoirs are phosphorus-containing rock stratums and deposits of inorganic and organic phosphorus compounds. Phosphates are usually not or hardly water-soluble and therefore unavailable for the plant in this state. The mineral enters the ecosystems by way of a stepwise degradation usually with the aid of microorganisms. The distribution of phosphates differs very much geographically: interesting deposits occur only in Morocco. Today, the prevailing view is that enough worldwide sources of phosphorus exist to supply the agriculture also in the future with enough fertilizer. It depends on the general political climate and on the price whether this optimistic prognosis comes true. Industrial nations can easily fund the import of phosphates, developing countries cannot.
Organic phosphorus sources are the guano hills before the coast of Peru that developed by accumulation of bird excrements and that are determinedly quarried since the 19th century.
The sulfur cycle is illustrated in the following picture. In contrast to phosphorus, sulfur is both oxidized and reduced by microbial processes. The plant can only use sulfates. Sulfates and phosphates are water-soluble and are thus easily eroded from the soil. A part accumulates in stagnant waters like lakes and ponds and contributes to their eutrophication. The main part of the phosphates is finally washed into the oceans where it is converted to insoluble compounds that accumulate at the bottom of the sea and are lost to the biosphere for the time being.
|
|