

From grade 4, Nick attended Heidelberg State School. His secondary education was at Macleod High School, where in year 12 Nick studied English, Chemistry, Physics, Pure Mathematics and Applied Mathematics. He was an accomplished athlete in his youth, specialising in middle and long distance events, including a sub 2.30 marathon.
Nick's passage through school was not entirely 'plain sailing'! When he was 15 years old he ran away to sea! He tried to organise an apprenticeship as a merchant sea officer, but that didn't work out and after several months he was persuaded to return to complete his secondary education. By the time he had done that the urge for a life at sea had decreased.
Nick studied at Melbourne University, where he completed a BAgSci and a PhD in Biochemistry.
Prof Hoogenraad has worked at:
Nick lists his hobbies as grape growing and wine making, gardening, carpentry and building, music, hiking, skiing, rock climbing and rogaining.
(Author's comment: Professor Nick Hoogenraad certainly puts the stereotype of a dusty, nerdish scientist to the test, doesn't he?)
Interviewer: Can you describe briefly how you are doing whatever you do?
Prof. Hoogenraad: Proteins have a "molecular address" on them. We isolate the gene (cDNA) encoding the protein and by genetic engineering we identify the part that is the address message. For example, removal of the part of the protein that is the address should stop targeting, but adding the DNA coding for this to another protein should make that protein go to the destination encoded. We are also isolating the genes for the molecular machinary at the end address so that only the correct protein enters that address or compartment. We clone these genes into bacteria, express them and characterise the proteins and the DNA.
Interviewer: Who or what (animal or plant) will benefit from your research/techniques?
Prof. Hoogenraad:In general terms, the misdirecting of proteins can be devastating. For example, if a protein goes to the wrong compartment, or fails to get to the correct compartment, that protein will probably not carry out its correct function. In humans, this can cause serious diseases ranging from cystic fibrosis to lactic acidosis or even death.
Understanding how this process works will lead to the ability to test for the defective genes as well as to treat the patients with targeting defects.
Interviewer: What are the economic implications of your applied genetics? (How expensive is it to do? What will be/are the cost benefits of the outcome?)
Prof. Hoogenraad: There is a great emphasis on producing valuable products such as blood clotting proteins, antibodies and hormones by genetic engineering techniques. This involves expressing genes in foreign cells, such as a mammalian gene in a bacterial cell. By judicious targeting of the protein to an appropriate destination in the cell it can be expressed in high levels and with optimum activity.
The production of antibodies for diagnostic purposes alone runs into hundreds of millions of dollars a year. Since most of these products are imported from overseas, production of high tech products such as these in Australia will not only create jobs, but it will have a significant impact on the outflow of Australian dollars to foreign countries.
Interviewer: Historically, what is the scientific background to the research you are now doing?
Prof. Hoogenraad: When we first isolated a cDNA for a mitochondrial protein (ornithine transcarbamylase) in 1982, we discovered that it had an extension on the end that was not present in the protein that we had isolated from liver cells. This extension proved to be the "molecular address" that targeted the protein to the mitochondria of the cell. When we removed this "address" the protein got lost in the cell, and when we joined this piece to a protein that normally stays in the cytosol it now finished up in the mitochondria.
This provided us with a model of a molecular postal delivery service, where there would be:
This whole topic has become a central theme in cell and molecular biology and it has been discovered that the Golgi apparatus is a "sorting centre" for the cell. Here proteins are sorted for:
Interviewer: For a Year 12 Biology student to understand what you are doing, some background knowledge of biological concepts will be needed. Can you tell me what biology is relevant to what you are doing, and why?
Prof. Hoogenraad:A basic understanding of the structure of DNA and the processes of transcription and translation is needed if students are to understand the relationship between the DNA base sequence of the gene and the primary structure of the final protein product.
During translation, the proteins to be targeted are made in the cytosol on ribosomes and they interact with proteins which carry them through the cell to the correct destination. The diagram below shows the problem facing a "new" protein at the ribosome; finding the correct location for its action without help would be practically impossible.
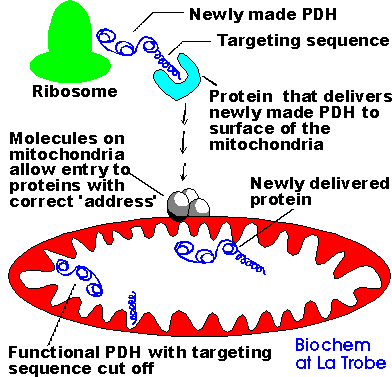
A key method in our investigation of targeting is to alter the cDNA encoding the protein we are studying. Essentially, we are creating mutations when we do this, either by deletions of segments or by transfer of segments to other cDNAs to make hybrid (chimeric) proteins. Students need to realise that alterations to the DNA such as these are going to lead to alterations in the protein product the DNA codes for. These mutations are used to confirm the importance of the targeting sequence or molecular address on the protein molecule in the targeting process.
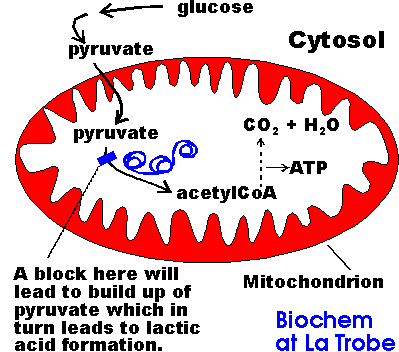
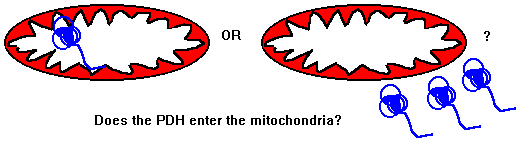
A number of clinical conditions have now been found which are caused by naturally occuring mutants. These result in a failure to target a molecule to its correct destination. An example is cystic fibrosis, where a mutation results in a failure to target a chloride channel protein to the plasma membrane of the cell. Another example is where a mutation in the address peptide of the enzyme pyruvate dehydrogenase (PDH) causes the enzyme to fail to enter the mitochondria. This, in turn leads to the build up of lactic acid in the cells, the lactic acid then enters the blood, causing lactic acidosis, brain damage, coma and eventually death. The normal function of the enzyme PDH is shown in the following diagram.
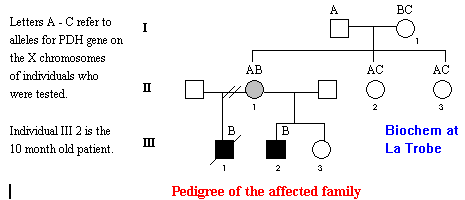
The genetics of the family with the mutation causing lactic acidosis is also interesting. The male patient was 10 months old when he was investigated at the Royal Children's Hospital Murdoch Institute for Research into Birth Defects. This child had a brother who had died at 33 months of age with what turned out to be lactic acidosis. The 10 month old baby was beginnig to show some similar symptoms and his mother was naturally concerned.
The scientists at the Murdoch Institute were able to analyse the DNA sequence of the area of the X chromosome which codes for PDH, not only in the baby, but also in many of his relatives, and in biopsy material from his deceased brother. In fact, they found four different alleles, which they called A,B and C, for the PDH gene in the family. The mother of the two boys admitted to some minor learning difficulties. It seemed that a mutation in the XB allele was the cause of the problem. The pedigree diagram below shows what was found out about the PDH alleles in this family. It shows that this X-linked disorder is expressed in the males who have only one X chromosome and so only carry the genetic information for the mistargeted PDH protein. In the boy's mother, who has two X chromosomes as she is female, her "good allele" (the XA allele) seems to code for enough normal PDH that the mutant allele on her other X chromosome causes her few problems. In this family the XB allele was found in the patient's grandmother, individual I 1, but not in her other two daughters, individuals II 2 and 3.
We were able to characterise the mutation as a G to C point mutation at nucleotide 134 of the PDH gene on the X chromosome. This mutation causes an arginine to proline substitution in the region of the PDH protein that we suspected would be involved in targeting. The mutation was present in the patient, his mildly affected mother and his deceased brother.
Interviewer: I now need to know something of the techniques used in your research. Can you tell me what techniques are important, and why they are important?
Prof. Hoogenraad: The flow chart which follows shows the basic steps of the process we used to investigate the targeting of the mitochondrial enzyme, pyruvate dehydrogenase. These techniques are routinely used in our lab in our continuing research into protein targeting within cells.





Add DNA polymerase and
dT, dA, dC, dG.
This is PCR to make many copies of the DNA. ![]()




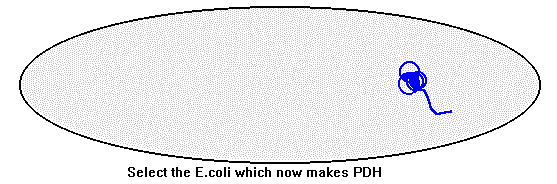
To isolate the cDNA which encodes the patient's PDH, the mRNA was first isolated from a sample of the patient's skin cells grown in culture. There are many different mRNAs in the skin cells at any time, as many different proteins are being produced, so we actually isolated all of them together. This mRNA mixture was used as a template to make cDNA, using the viral enzyme reverse transcriptase. This gave a mixture of cDNAs. The cDNAs were inserted into phage vectors which were used to infect bacteria (E.coli). This created a library of cDNAs from the patient, representing all of the mRNAs originally isolated from his skin cells.
To find the E.coli with the cDNA we were interested in antibodies were used to detect the PDH protein made by these E.coli. The antibody was made by collecting PDH from human tissue, purifying it, and injecting it into a rabbit. The rabbit's immune system produced antibodies to the "foreign" human PDH protein. This became the probe to screen the gene library in the E.coli for the bacteria making the PDHprotein.The base sequence of the cDNA from the patient was determined next, using a sequencing gel. By comparing the patient's sequence with that of the PDH cDNA from a normal person we were able to identify the mutation in the patient. It was a point mutation with a cytosine substituted for a guanine. The effect of this mutation is to replace an arginine with a proline in the protein product of the gene. In fact we found that the mutation was in the part of the cDNA which is thought to code for the enzyme's mitochondrial address signal. So we decided to test if the mutation caused the protein to be mistargeted.
Proof that the mutation caused the patient's problem was obtained by making the mutant protein in vitro and showing that it could not be targeted to the mitochondrial compartment. To do this the cDNA for PDH was used as a template for production of mRNA, using the enzyme RNA polymerase in vitro with the four ribonucleotides and suitable conditions of temperature and pH. Once we had the mRNA we used it to make the PDH protein in vitro. For this we needed to add the complex translation machinary of ribosomes, tRNAs and all the necessary amino acids. We added one radioactive amino acid instead of the normal form, so we made a radioactivelly labelled protein which we were able to track in later experiments.
We used freshly isolated mitochondria from liver cells to test our PDH proteins. In the case of the PDH from the patient, it was not imported into the mitochondria. The PDH from a normal person was successfully imported. To confirm that the problem with the patient was the inability to target PDH to the mitochondria we swapped the defective targeting signal with a normal one from another mitochondrial enzyme and placed the defective signal onto the other enzyme. Now the patient's PDH was imported into the mitochondria correctly but the other enzyme could no longer be imported.
The "cutting" and "pasting" of pieces of protein like this is done at the level of the DNA. In a very real sense, this is "genetic engineering" although we also call it "protein engineering". We use PCR to amplify the DNAs, in this case from the patient and from a normal person, then restriction enzymes and DNA ligase to cut and paste any pieces of DNA for which we know the sequence. The recombinant DNA can then be used in vitro to produce the new protein we are planning to investigate.

Here is one of Prof. Hoogenraad's PhD students, Joe, showing us the PCR machine. It's interesting to see that such a high-tech process which makes many copies of a piece of DNA can be carried out in such a small machine.Interviewer: What do you see as the major biological implications of the work you are doing? For example; are genotypes or phenotypes being altered? Is a species survival potential increased? Will someone or something have a better quality of life?
Knowing the genetic basis for a problem such as the PDH mutation which leads to lactic acidosis opens up the possibility for genetic screening for this disorder. This leads to accurate diagnosis of the cause of the abnormality. It may also allow us to modify diets to minimise clinical symptoms and thereby prevent permanent damage. In fact the patient we have discussed is being carefully monitored and managed through the difficult development period of his childhood.
Gene therapy is the only ultimate solution to problems such as this. Once the genotype can be corrected the individuals with such mutations will be able to lead normal lives.
Interviewer: There is often discussion or debate about issues associated with biotechnology. What is one such issue relating to your work? Can you outline the arguments of the opposing sides of the debate please?
Prof. Hoogenraad:The development of technology is expensiveand someone has to pay for it. On one hand, as an ever increasing number of diagnostic tests become available, the cost of medicine is increasing quite alarmingly. The more sophisticated treatments we have, the larger the proportion of the country's resources we will spend on medicine.
On the other hand, we have a justifyable expectation that we will get the best treatment available. One outcome of the "biotechnology revolution" is that we can now make therapeutic reagents more cheaply than before. For example, once expensive hormones extracted from tissues cnanow be made by "genetic engineering" techniques as we have discussed. A very positive outcome of using bacteria as factories for making therapeutic reagents is that it eliminates the possibility of accidently isolating a contaminating virus such as HIV, as occurred with blood products. Also if we can treat a person to prevent serious effects from occurring, such as brain damage, we will certainly save a great deal of the cost of supporting such a person.
Then there is the question, "should we test for anything at all?" If we could test at birth for the possibility of getting a disease in middle age, would we want to do this? Would we want to know the answer? Who would tell the child? And when? And if we did know, who else should have this information? Your insurance company? Your potential spouse or partner?
These are all questions that governments, insurance companies and individuals will face as the technology for testing and treatment of genetic disorders becomes more widely available.
I am sure that there would be discussions of these topics on the Internet, as many of these questions arise out of the Human Genome Project as well as out of our research. There is a section at their Web site where ethical, legal and social issues are discussed.
Interviewer: Can you suggest some reading material relevant to anything we have discussed about your work, its implications and issues associated with it?
Prof. Hoogenraad: Students should be able to find information relevant to this topic in publications such as New Scientist and Scientific American, which both have Internet sites as well as the magazines. The Age has a CD ROM of the last 5 years of the newspaper, where issues associated with the new gene technologies are discussed peroiodically. And a search of The Internet would undoubtedly find many articles at a variety of levels, about this topic.
(Interviewer's notes:
1. The scientific paper in which this investigation was reported may be obtained by your school librarian, but BE WARNED that most of it is very technical and students and their teachers, including this teacher, would find much of it difficult if not impossible to understand. However, if you are interested, the reference is:
Fumie Takakubo, Peter Cartwright, Nicholas Hoogenraad, David Thorburn, Felicity Collins, Trevor Lithgow and Hans-Herik M. Dahl, "An Amino Acid Substitution in the Pyruvate Dehydrogenase EI alpha Gene, Affecting Mitochondrial Import of the Precursor Protein" American Journal of Human Genetics, 57:772-780, 1995.
2. The well known science writer, Graeme O'Neill, interviewed Prof Hoogenraad in 1996 about some of his work. That interview focuses on protein folding and what can go wrong with it. The interview is reproduced as another page of the VCE Biology site. It is not directly relevant to the interview reported here for CAT 2, but is all part of the work done in Prof Hoogenraad's group. The article is titled 'Molecular Chaperones, Guardians of Life'.
Interviewer: Thank you for your time.For ideas on where you might find additional resource material, look again at the section Resources on another page at this site. And don't forget the power of the Internet to provide you with information. A well thought out search, using a search engine such as Alta Vista, should provide more information than you can possibly use!
Please do not phone Prof Hoogenraad for further information for your CAT. He, like the rest of the staff in Biochemistry, is a busy person. However, if you think we can help you some more, use either the comment form or the email address for this site and we will do what we can.
If you would like to read another interview with a La Trobe Biochemistry scientist, you can click here to go back to the Interview Index.
If you would like to 'netsurf' for some more resources, you might find the author's favourite Biology Bookmarks helpful.