Lecture 5Free energy changes in biochemical systems |

In this equation, the term DGo provides us with a value for the maximal work we could obtain from the reaction starting with all reactants and products in their standard states, and going to equilibrium. The term DG' provides us with a value for the maximal work we could obtain under the conditions defined by the activities in the logarithmic term. The logarithmic term can be seen as modifying the value under standard conditions to account for the actual conditions. In describing the work available in metabolic processes, we are concerned with the actual conditions in the reaction medium (whether that is a test-tube, or the cell cytoplasm); the important term is therefore DG'. If we measure the actual activities (in practice, we make do with concentrations), and look up a value for DGo in a reference book, we can calculate DG' from the above equation.
Values for DGo provide a useful indication through which we can compare the relative work potential from different processes, because they refer to a standard set of conditions.
An example of the former case is provided by reactions involving nucleotide phosphates such as ATP, ADP, GTP, GDP, etc. These reagents act as chelating groups for bivalent cations such as Mg2+ or Ca2+, and the species undergoing reaction is often the metal-complex form. In any case, the concentration of the active species will differ from the total estimated by standard biochemical assays, because of the mixture of free and metal-complexed forms of the reagent.
An example of the latter case is the activity of the H+ in protolytic reactions (those giving rise to an uptake or release of H+). The pH inside a cell is close to neutral (pH 7.0, or {H+} = 10-7M). The value for DGo for such a process could be RT x ln(7) different from the value at pH 7.0, since the standard state is 1M.
If we attempted to include all such complications in our equation for a metabolic reaction, we would end up with a very unwieldy set of equations. In order to avoid this, biochemists have adopted a useful convention. The parameters which can be readily measured by biochemical or chemical assay (for example, the total concentration of reactants) are included in the logarithmic ratio. All other terms, which will vary with conditions, are taken out of the logarithmic ratio, and included in a new reference free energy, by adding parameters to DGo. This new term is given the symbol DGo', and its value is specified with respect to the particular conditions which determine the additional free energy terms changing DGo. What we end up with is a new equation, which looks like the one above, but has a slightly different meaning:
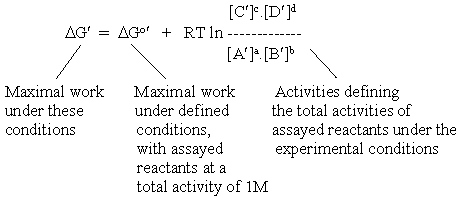
It is obviously important to chose a value for DGo' appropriate to the reaction conditions. Fortunately, the literature provides graphs and tables for such values, from which the experimentalist can chose, or obtain an appropriate value by interpolation (see examples for the case of ATP hydrolysis).
In the examples from glycolysis above, the values for free energy change are DGo' values under the "biochemists" standard conditions of pH 7.0, 25o C.
The same general principle is used in the thermodynamic description of redox reactions. See later sheets for a discussion of the relation between DGo' and DEo', and a more detailed treatment of the effects of H+ and other ligands in the context of redox reactions.