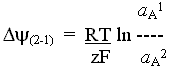Lecture 13A brief overview of methodology
|
Estimation of the proton gradient
The proton gradient has contibutions from electrical and chemical terms:
Dp = Dy - ZDpH
The contributions can be assayed by use of ionophores or similar reagents.
The membrane potential component, - Dy
In general, the methods used depend on the Nernst equation describing the condition for equilibrium distribution of an ionic species between phases of different electrical potential.
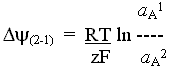 The conditions that must be met are:
The conditions that must be met are:
- The ion being measured must be at electrochemical equilibrium. This will be achieved if the ion can cross the dividing barrier rapidly.
- It must be possible to measure the concentration of the ion in both phases.
- All competing fluxes must be negligible.
In practice, condition 1 is achieved either by use of valinomycin and one of the monovalent cations which it transports rapidly (K+, Rb+, Cs+), or by using a lipophilic ion.
Condition 2 is achieved by using isotopic labelling (86Rb, or 14C-labelled organic lipophilic cation or anion). It is necessary to separate a reaction mixture into vesicular and bulk components (usually by centrifugation), measure the amount of ion in each fraction, measure the water volume associated with the bulk and enclosed phases, and calculate the concentrations. In practice, this is done by using tritiated water (which distributes to all phases), and a soluble but membrane impermeant marker (say 14C-labelled sucrose), and finding the sucrose impermeant space by subtraction, then allowing for distribution of the measured ion into the bulk phase of the pellet.
Condition 3 is much more tricky, and is not easily demonstrated. Usually, a kinetic compromise is used,- the distribution is measured at a time when the condition of 1 is achieved, and nothing much else has happened!
The chemical component, - DpH
Measurement of DpH depends on the equilibrium distribution of either:
- 86Rb-salt in the presence of nigericin to catalyze the neutral exchange of Rb+ for H+.
[Rb+]L / [Rb+]R = [H+]L / [H+]R
- The distribution of weak amine cation:
[RNH3+]L / [RNH3+]R = [H+]L / [H+]R
This approach has been used extensively with fluorescence amines (9-aminoacridine, atebrin), especially with chloroplasts, where H+-uptake occurs, with concentration of the cationic form in a the lumen. This leads to self-quenching, and therefore a fluorescence change related to the DpH. The relationship is not linear, but provides an assay which is relatively easy and quick.
- The distribution of the anion of a weak acid:
[A-]R / [A-]L = [H+]L / [H+]R
This approach was the original technique used to assay DpH in mitochondria where protons are pumped out, so the anion is accumulated.
For methods using isotopic labelling, the problems of measurement of concentration in the two phases are similar to those mentioned under estimation of membrane potential above.
The oxygen electrode, and its use in measurement of respiration and photosynthesis
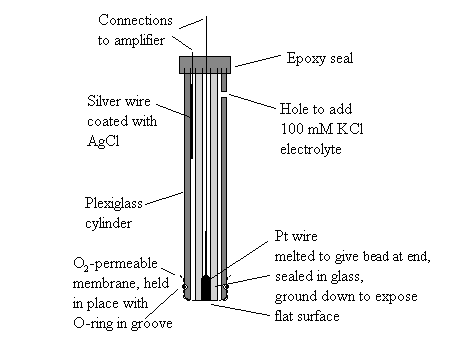
The oxygen electrode is a simple device in which a current proportional to [O2] is generated by polaarization of a Pt-electrode to reduce the O2. The configuration used for measuring respiration or photosynthesis is that suggested by Clark (the Clark oxygen electrode), and available in many commercial forms. In this configuration, the Pt-electrode area is kept small, so as to minimize the rate of consumption of oxygen, and the Pt and reference electrodes are separated from the reaction medium by an oxygen permeable membrane. You can build one yourself for a few $$$ or buy one for $1000s.
A specialized configuration,- the oxygen rate electrode,- is used to measure the flash yield of oxygen in photosynthesis. The Pt-surface is made much larger, so as to maximize the current at low oxygen concentration. Chloroplasts or algae are applied in a thin concentrated layer, and the oxygen produced on flash illumination is measured by integrating the area under the transient.
Redox potentiometry
See notes from lecture 7.

©Copyright 1996,
Antony Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu