The research into all cell structures was and is dependent on microscopic techniques. Electron microscopy helps to study the substructures of organelles already known by light microscopy and extends even into the molecular range. But it can only be used for the examination of fixed and contrasted, in other words, dead cells and is therefore unsuitable for the depiction of intracellular movements or transport processes. These can be studied with advanced techniques of light microscopy, like UV-microscopy, AVEC-DIC and fluorescence microscopy with nonpoisonous dyes. The use of UV microscopy is especially interesting when studying bundles of actin filaments that cannot be studied with electron microscopy due to the lack of a suitable contrasting agent. AVEC-DIC has become the method of choice for the research into intracellular movements and cytoskeletons. Fluorescence microscopy of living cells can be used to examine the membranous flow.
The complete repertoire of subcellular organelles and other inclusions was made visible by electron microscopy. But nothing living can be seen in an electron microscope. The nearest you get to the study of dynamic processes within the cell's plasma is the comparison of picture series. Several light microscopic techniques have been optimized, new extremely specific fluorescence dyes have been developed and the use of video cameras and computers optimized the documentation and evaluation of the pictures. Three of these methods shall be shortly reviewed here: UV-microscopy, AVEC-DIC and the use of new fluorochromes.
UV-microscopy offers two principal advantages. On one hand UV light has a shorter wave length (less than 310 nm) than visible light (lambda = 400-800 nm), thereby reducing the resolution limit of ABBÉ to about 0.1 Ám. On the other hand many subcellular structures absorb ultraviolet light due to their amount of proteins and nucleic acids and can thus be perceived even without additional contrasting.
Still, UV-microscopy did not gain general acceptance for a long time. The cause were the difficulties in the documentation of results. Not before the use of video cameras showed UV microscopy to advantage (I. K. LICHTSCHEIDL and W. G. URL, Institut für Pflanzenphysiologie, Universität Wien, 1987). Beside the cell nucleus it is possible to identify mitochondria, plastids, spherosomes, the Golgi apparatus and other structures without doubt. Additionally tubular elements of a diameter of 100 nm or more can be seen that could upon comparison with electron and fluorescence microscopic studies be shown to be parts of the endoplasmic reticulum. In the quiet plasma near the cell wall they can often be perceived as a polygonal network, while they are parallel to the current's direction in plasma currents.
Furthermore bundles of actin filaments can be depicted, that are impossible to detect in electron microscopic images due to the lack of a specific contrasting agent. In single UV microscopic images they can only hardly be distinguished from tubular ER. But their involvement in movements shows, that they are structures other than the ER. Additionally they can definitely be identified with special fluorochromes in fluorescence microscopy (see the following section).
N. S. ALLEN and R. D. ALLEN developed Allen's video-enhanced contrast, differential interference contrast technique named after them. It is based especially on the image evaluation of interference contrast microscopic takings. The pictures taken by a video camera can be digitalized and thus be computer-analyzed. Upon subtraction can, for example, all identical structures in successive images be erased. As a result only all the moving particles are depicted. But it is also possible to subtract images of different depth of field planes, so that a background, which is in the way, can be filtered out. The technique has become the method of choice for the study of cellular or intracellular movements and the cytoskeleton during the last years.
N. S. ALLEN and R. D. ALLEN (1978) as well as I.K. LICHTSCHEIDL and D.G. WEISS (1987) have used the technique for the examination of plasma movements in plant cells (Chara, Allium cepa, and others). Its advantages are only poorly reflected in the pictures shown here. Only in video films the whole range of movements within the plasma can be properly portrayed. By evaluation of the films the different speeds of movement of the different particles can be calculated. It can thus be shown that certain particles 'walk' along bundles of actin filaments and change their direction as soon as they reach a bundle with another polarity. It shows also that the ER functions as a mechanic obstacle for the movement of some particles.
The lipophilic cation 3,3-dihexyloacarbocyanin-iodide (DiOC) proved to be the ideal marker for the ER and other membranous systems (H. QUADER and E: SCHNEPF, Zellenlehre, Universität Heidelberg, 1986). Since the cells are not fixed during this procedure and since the fluorochrome does not - at least for some time - impair the cellular functions, is it well-suited for the study of the membrane flow in living cells. This is different with fluorescence-marked phalloidine, the poison of the amanita that turned out to be actin specific and can thus be used for the detection of actin filaments in fixed cells. Actin filaments are particularly common in the area around the nucleus and from there they expand across the whole protoplast. They are combined in thick bundles or cables near the nucleus, but the further they expand from the nucleus, the more do they split up.
The newest technique of light microscopy is confocal laser scan microscopy, where the specimen are captured layer after layer and are analysed with the aid of a special software. The depiction of fluorescence-tagged, moving objects, for example that of elements of the endoplasmatic reticulum, other membrane systems or the cytoskeleton, are very impressive. It shows that the ER is tightly coupled to bundles of microfilaments or that dictyosomes move along the filaments as if on rails. An article with film sequences by Cris HAWES (Oxford Brooks University) can be found at the following internet site:
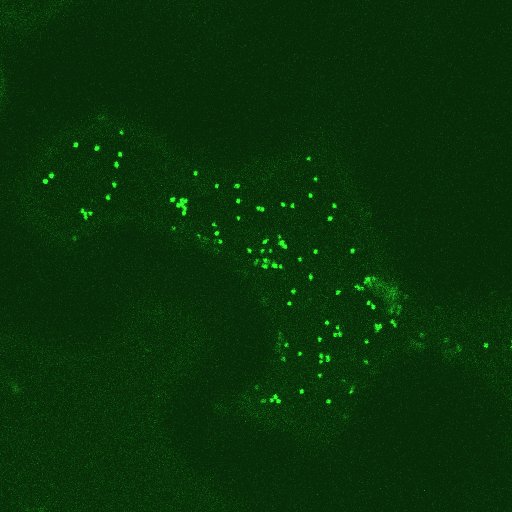
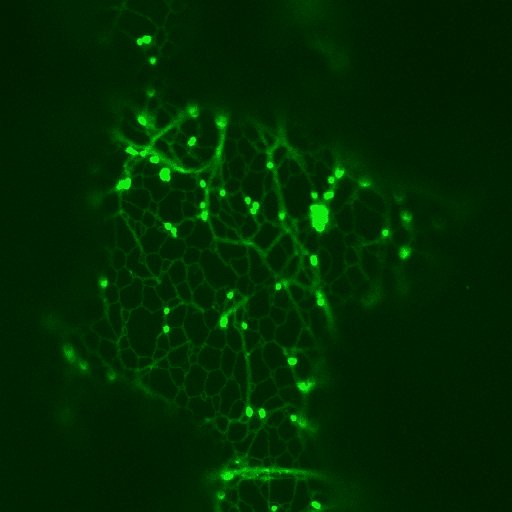
Stacks of Golgi-vesicles und cortical ER network in leaf epidermal cells - labelled with GFP ("green fluorescent protein": The Golgi-stacks show a striking, the ER in the right picture a weak green fluorescence. For details see the feature by C. HAWES et al. The Golgi stacks are highly mobile, they are associated with the ER and travel along the cortical ER ribbons. The movement is both uni- and bidirectional along a strand of ER. This novel close association can best be seen in movies. - The association of ER-network , Golgi-stacks and the actin network will be described elsewere
Click in the pictures to activate AVI-files.
To activate or stop movement click into the AVI-pictures
©
C. HAWES.
|
|