Translation is the process during which an mRNA's sequence of bases is translated into a sequence of amino acid. We already got to know the most important components of protein biosynthesis: mRNA, tRNA, amino acids, ATP, GTP, magnesium ions, aminoacyl-tRNA synthetases and 'factors' (see below). Additionally, ribosomes are necessary.
No structural relationship exists between codon and its respective amino acid. Consequently, an adapter, a tRNA, is necessary to bind the amino acid and to recognize the respective codon. In a tRNA structure is / are:
- the anticodon always found at the same place, at the end of one of the loops.
- the bases of the anticodon 'free'. i.e. they are not paired with other bases of the tRNA.
- the amino acid linked to the 3'-terminus of the tRNA, opposite of the anticodon.
- a clearly defined tertiary structure found. The molecules are of a compact form.
The mentioned properties are the preconditions for
the adapter function. Due to the compact form, several tRNAs can bind
next to each other to a mRNA so that codon after codon is bound. Such
associations cannot take place in solution due to the lack of
stability. To form a peptide link between two neighbouring amino
acids, they do have to be very close to each other. Since one or
several enzymes are unable to perform this task, the surface of a
large supramolecular structure, the ribosome, is needed.
A mRNA binds to a ribosome. Amino acid specific tRNA molecules (squares) bind the respective amino acids (with the help of specific aminoacyl transferases). The amino acid-tRNA complex is bound via codon-anticodon recognition to a ribosome. The amino acid is transferred to a growing polypeptide chain. The amino-acid-free tRNA is given back into solution.
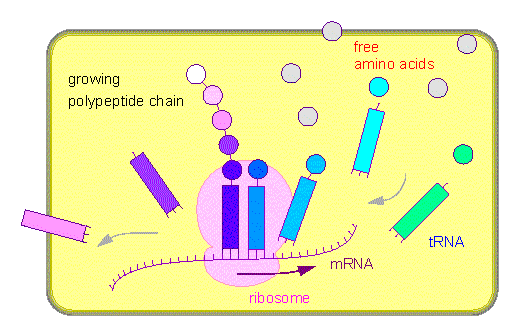
Ribosomes
consist always of two
subunits, a large and a small one. Each of
them contains rRNA (1-3 molecules) and a number of different
proteins. Ribosomes from Eschericia coli have been studied
most. Eucaryotic ribosomes (from the cytosol) are larger than
procaryotic ones. Those of mitochondria and chloroplasts resemble
procaryotic ribosomes.
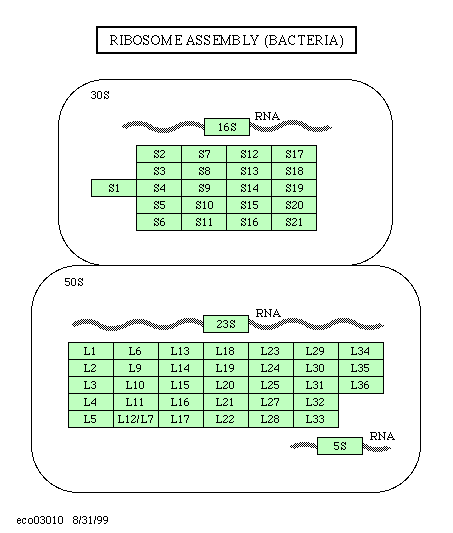
Clickable map - next level: amino acid and
nucleotide sequences of ribosomal proteins and rRNA.
Ribosomal structures of other
species in following list. Links connect to the original URL in
Kyoto).
The synthesis of a polypeptide chain occurs catalytically. The already mentioned 'factors' are proteins that are needed for the initiation, elongation and the termination of the polypeptide chain. One to three factors participate in each of these processes. Eucaryotic and procaryotic factors differ fundamentally.
Protein synthesis is a rather energy-consuming process. Besides ATP, GTP is required. A large portion of the energy is used for proof-reading. The newly synthesized peptide starts folding even before it is ready. It develops a secondary and a tertiary structure and achieves thus a thermodynamically favourable state. After synthesis is the chain often modified. Some parts are removed, some amino acids are changed, carbohydrates or lipids are added or the peptide is activated by acetylation, methylation or phosphorylation.
Part of the new proteins remains in the cytosol while others are exported through the membrane or imported into mitochondria or chloroplasts.
All these changes are collectively termed post-translational modifications or modulations.
For the binding of a mRNA to a ribosome is a specific nucleotide sequence that precedes the coding sequence necessary. Due to the differing recognition signals, factors and other control mechanisms is the translation of a eucaryotic mRNA with procaryotic ribosomes impossible (neither does it work the other way round). This is also the reason why no eucaryotic RNA could be expressed in the NIRENBERG-MATTHAEI system described at the beginning of this topic. Expression was only successful after developing the system with wheat germs. The example shows how specific translation mechanisms are. The situation is similar with transcription.
All these mechanisms (including post-translational modifications) together are called gene expression. The numerous steps required and the large amount of consumed energy reveal how important this process is for the basic metabolism of the cell. 88 % of an Eschericia coli cell's total energy turnover is necessary for protein biosynthesis. The expense is understandable when realising that the products (proteins, enzymes, regulators) have key functions in the cell's metabolism: the trouble-free operation of the single metabolic pathways is dependent on their function. Still, the error rates are by far larger than those of replication. A multitude of mRNA and protein copies per gene are produced during transcription. Each cell can survive if some of them are faulty. But just one molecule is doubled during replication. Each daughter cell receives one copy and each occurring mistake (mutation) is irreversible and will be inherited by all subsequent cells of this line.
The many steps of gene expression allow controls at different levels. Differentiation is achieved by differential gene expression. This means that the signals contained in the DNA or the respective mRNA (nucleotide sequences, secondary structures) determine whether a gene is expressed more often than another or that one set of genes is expressed before another during ontogenesis. Changes may occur abruptly or fluidly.
The binding of a mRNA to a ribosome, for example, is dependent on its recognition sequence. But not all types of mRNA are equipped with the same sequence. Consequently are those with recognition sequences with the highest affinity bound most.
At low affinity is the probability that a mRNA is bound by a ribosome low, too, and as a consequence are only few protein molecules produced. But this is just one of many possibilities to determine the number of protein molecules per cell. A further control mechanism is, for example, the affinity between mRNA and initiation factors, a third is the half-life of the mRNA.
Translation, just like transcription, works according to an assembly line principle. As soon as part of the mRNA is set free by the ribosome is it bound by a further ribosome so that the synthesis of a second polypeptide chain can already be initiated before the first is finished. In this way can a number of polypeptide chains be produced simultaneously from the same template mRNA. Numerous ribosomes are involved. Such complexes are called polysomes.
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de