The observation that a willow that has been cultivated in a container for five years with enough watering gained more than half a centner weight although only two ounces of the container's soil were lost goes back to J.B. van HELMONT (1577 - 1644). The British natural scientist S. HALES (1677 - 1761) understood that air and light are necessary for the nutrition of green plants. But it was not before the composition of air out of different gases became known that their significance for plant nutrition was studies. In 1771 observed J. PRIESTLEY (1733 - 1804), one of the discoverers of oxygen, that green plants give off oxygen and thus improve the air.
The priest J. SENEBIER (1742 - 1809) from Geneva discovered that the regeneration of the air is based on the use of 'fixed air' (carbon dioxide). These observations were confirmed and broadened by studies of the Dutch doctor J. INGENHOUSZ (1730 - 1799) who recognized both the meaning of light and the fact that the whole carbon contained in plants is of atmospheric origin. He, too, conceived that plants take up small amounts of oxygen at night or in the shadow and give off carbon dioxide. In 1804 discovered Th. des SAUSSURE (1767 - 1845) from Geneva that the plants' increase in weight cannot solely be caused by the uptake of carbon and minerals, but is based on the binding of the water components, too.
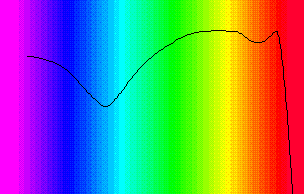
In 1894 constructed T. W. ENGELMANN (1843 - 1909) a gadget out of a modified microscope condenser that allowed him to expose parts of photosynthetically active cells (of the green alga Spirogyra) to a thin ray of light. His aim was to discover which components of the cell functioned as light receptors. To measure the oxygen production, he dispersed the thread-like Spirogyra in a bacteria-containing suspension. Whenever parts of the chloroplast were illuminated, did the bacteria concentrate in this area (where oxygen was available). The illumination of other parts of the cell resulted in no such aggregations.
In an earlier study did he split white light into its spectral components using a prism. He then illuminated a green alga, Chladophora, with this spectrum. In contrast to Spirogyra are the Chladophora cells completely and evenly filled by the chloroplast. He observed that the bacteria accumulated mainly in the blue and red light. A first action spectrum of photosynthesis was thus yielded. It resembles roughly the absorption spectra of chlorophyll a and b.
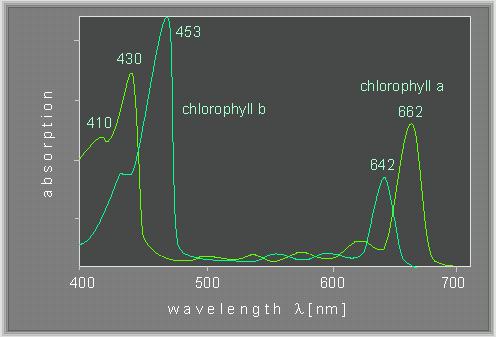
J. v. SACHS (1832 - 1897) could finally prove that chlorophyll is involved in photosynthesis. In addition did he show that starch is produced in chloroplasts as a result of the photosynthetic activities.
These results are in accord with the first law of thermodynamics, whose discoverer J. R. MAYER postulated already in 1842 that plants take up energy in the form of light and that they transform it into another, a chemical state of energy. Based on this assumption was the reaction equation
6 carbon dioxide + 6 water > (chlorophyll) > glucose + 6 oxygen
J.v. LIEBIG assumed that the oxygen stems from the breakdown of the carbon dioxide. This idea was uncritically accepted by the plant physiologists of the late 19th and the early 20th century (SACHS, PFEFFER, JOST and others) although M. J. SCHLEIDEN had as soon as 1842 realized that
- glucose is produced as a result of photosynthesis (and he was closer to reality than SACHS was later) and that
- it is very likely that it is water that is broken down. He wrote:
"It is well-known that CO2 is among the most stabile compounds and that no chemical way of breaking it down is known while H2O is very easily broken down.... and it does therefore seem likely that the 24 H2 of the 24 H2O are combined with the 12 CO2."
(from: Grundzüge der wissenschaftlichen Botanik). SCHLEIDEN's equations contain all reaction compounds in double numbers. He gives C12H24O12 as the formula for glucose.
It was soon realized that the reaction equation above is a simplification and that photosynthesis consists of a number of partial processes.
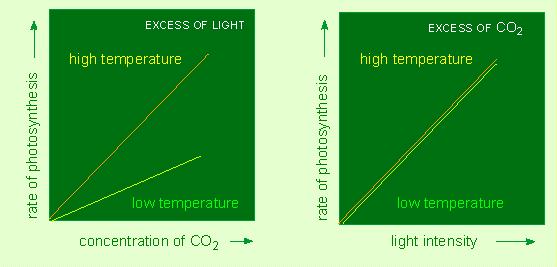
F. F. BLACKMAN and G. L. C. MATHEI (1905, University of Cambridge, Great Britain) were among the first to study this topic systematically. They cultivated plants under different but controlled carbon dioxide concentrations, different light intensities and different temperatures and they noted the effects of these parameters on the rate of photosynthesis. Two decisive aspects were revealed. Under strong light and limited amounts of carbon dioxide is the rate of photosynthesis dependent on the temperature. This shows that the carbon dioxide fixation is based on normal, temperature-dependent biochemical reactions. Under carbon dioxide excess and too little light was no temperature-dependence found. This hints at the fact that the light-induced reactions are independent of the temperature. This statement applies to all photochemical reactions.
In 1925 put O. WARBURG (Kaiser-Wilhelm-Institut [later Max-Planck-Institut] für Zellphysiologie at Berlin-Dahlem) the results of BLACKMAN down to the existence of two classes of photosynthetic reactions: the light and the dark reactions.
During the thirties analyzed C. B. van NIEL (Stanford University) the photosynthesis of a number of purple bacteria. In addition to carbon dioxide do these bacteria need hydrogen sulphide for photosynthesis. van NIEL was able to determine
6 CO2 + 12 H2S > (light) > C6Hl2O6 + 12 S + 6 H2O
as the reaction's equation. Based on it did he extrapolate a general equation of photosynthesis:
CO2 + 2 H2X > (light) > (CH2O) + H2O + 2 X
According to this equation is photosynthesis a redox reaction with H2X as the electron donator (the oxydizable substance). In the case of green plants is it H2O and this means that not the carbon dioxide but the water is broken down.
A first experimental prove that the oxygen developed during the photosynthesis of green plants stems indeed from water was delivered by the British physiologist R. HILL. He detected that isolated chloroplasts give off oxygen in the presence of unnatural reducing agents like iron oxalate, ferricyanide or benzoquinone after exposure to light. The reaction went down in literature as the HILL-reaction:
2 H2O + 2 A > (light, chloroplasts) > 2 AH2 + O2
where A is the electron acceptor. If A = FeIII, then is
2 H2O + 4 FeIII > (light, chloroplasts) > 4 FeII + O2 + 4 H+
The process is linked to a photolytic breakdown of water that precede the reduction of FeIII.
4 H2O > (light, chloroplasts) > 4 H+ + 4 OH-
| oxygen can also be set free in the absence of carbon dioxide, | |
| the oxygen produced stems from the breakdown of water, | |
| isolated chloroplasts are able to perform at least partial processes of photosynthesis. |
The statement that the oxygen produced during photosynthesis stems only from the breakdown of water was confirmed by S. M. RUBEN, M. RANDALL, M. KAMEN and J. L. HYDE in 1941 after the isotope technique had found its way to biochemistry. They could shown that a suspension of Chlorella grown in H218O, gives off 18O2, after light exposure. Shortly afterwards confirmed S. M. RUBEN and his collaborators the postulate of O. WARBURG that the fixation of carbon dioxide is energy consuming but independent of light. In addition could E. RACKER (Cornell University, Ithaca, N. Y.) prove that light can be replaced by the addition of energy-rich compounds.
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de