Growth- and differentiation-processes are collectively called development. They are characteristic for all organisms. Cell differentiation starts in plants directly after the establishment of polarity. In plants is gene activation especially controlled by endogenous and exogenous factors meaning that control genes and environmental factors influence the expression of numerous structural genes alike. Rarely is such a control about all-or-nothing reactions. Instead does it usually increase or decrease the transcriptional activities. A change towards a certain specialization does not mean that the whole former program is stopped and exchanged against a new one but that the ratio of produced enzymes, other structural proteins, and RNA towards each other is changed.

Activity Changes of Enzymes Involved in Purine Recycling. Measurements were carried out on coleoptiles of lupin directly after germination. 1: Adenosine kinase, 2-4: Phosphoribosyl transferases, 5: Adenosine nucleosidase, 6. Nucleoside phosphotransferase (C. WASTERNACK, 1982)
Before one (or several) phytohormones can begin to control the development of certain processes have the enzymes required for hormone synthesis to be produced or activated. Additionally has the expression of those genes whose products are responsible for a cell's sensitivity towards a hormone to begin. The regulation at the transcriptional level and at that of the nearly complete protein is of no smaller importance. Activation, for example, can be achieved by a changed ion milieu, binding of co-factors, phosphorylation, or by the proteolytic cleavage of a terminal peptide.
The activation of an existing enzyme is characterized by a linear reaction kinetics (as a function of time). The activation of amylopectinase during seed germination is an example. In contrast can the initiation of a de novo-synthesis be recognized by a sigmoid reaction kinetics.
The delay during the start (the lag-phase) corresponds to the period of time required for the enzyme's production. The synthesis of alpha-amylase, for example, is induced by seed germination.
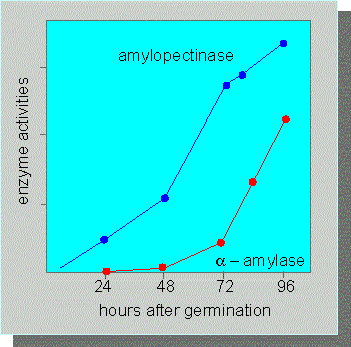
The activity of some enzymes of Pisum sativum (amylopectinase, for example) increases fast after germination. Transcription inhibitors like chloramphenicol do not influence the increase in activity. Consequently has the enzyme or its mRNA already to be present during germination. Other enzymes like alpha-amylase turn up much later. Their appearance can be prevented by the addition of chloramphenicol, which means that their synthesis begins after germination (Y. SCHAIN, A. M. MAYER, 1968).
The development (ontogenesis) begins with the subdivision of the embryo into organ anlages. They develop into organs, in which cells combined in tissues specialize even further. The development of a higher plant can therefore be described by three stages:
| the juvenile stage that starts with the germination of the seed, | |
| the stage of growth and maturation; it covers the vegetative growth including the development of the organs and tissues and ends with the reproductive stage. | |
| the reproductive stage and senescence |

As has been mentioned at the beginning can several extern factors induce seed germination if the seed itself is primed. Germination does thus start at the most favourable time of the growing season. It interrupts seed dormancy. In areas with seasonal variations of temperature and periodically changing day lengths are the control mechanisms simple and reliable (photoperiodism), while the rare and sporadic rainfall of deserts requires other strategies. In such areas exist species whose seeds start to germinate only after a certain threshold of rain days has been reached. The thresholds differ individually so that not all seeds germinate after the first days of rain. The ones that germinate early use every rainy day for their development while those with a higher threshold value wait for possible better times.
In dormant seeds occurs no DNA replication and protein biosynthesis works at a hardly detectable level. After germination - that is nearly always linked to an increase in volume due to water uptake of the cells - begin numerous metabolic activities. The synthesis rate of nucleic acids and proteins rises rapidly, the activity of the required enzymes changes dramatically and after exposure of the shoot bud to light begins the synthesis of chlorophyll. The proplastids change into plastids and photosynthesis begins.
During growth reach the metabolic processes their optima. As a
consequence increases biomass rapidly. Growth is therefore always
linked to an irreversible increase in weight. The cells multiply
and increase in volume. Secondary metabolism starts usually when
the primary metabolism (especially photosynthesis) runs at top
speed and enough assimilates required for further use have been
accumulated. All regulated growth-activities and metabolic processes
go through an exponential stage of activity increase. It is followed
by a phase of saturation where hardly any increase occurs until
growth as a function of time reaches asymptotically a stop. Several
parameters indicate the drop in activity. The most striking is
maybe the reduction of the leaf size. In each shoot are the lowest
leaves that have developed first larger than that at the tip.

Growth Rate; the activities of physiological reactions and of the temperature are coupled.
We discuss the transition to the reproductive stage in more detail in the context of flowering hormones. Flower formation itself consists of three stages:
- induction,
- initiation of a flower meristem and
- anthesis, i.e. opening of the flower.
Different species have different mechanisms with which they control the development of their flower organs (stamens, carpels). You will find more about this in the next section. In most species develop carpels and stamens simultaneously, but in some grow the male flower organs first (protandrous flowers) and in still others the female (protogynous flowers). In a few species like pumpkin (Cucurbita pepo) develop male flowers first, then hermaphroditic ones, finally female and at last parthenocarpous flowers that produce seeds agamically.
Senescence starts together with flower development. During this stage are assimilates mobilized and used for seed development. Moreover are they fed into perennating organs (if existing). The vegetative organs stop their metabolic activities successively and start to wither unless they are perennial. Withering parts fall off.
The process described so far is mainly valid for annual plants, though part of it is also true for perennial plants. The organs of a perennial plant like a tree, for example, can be grouped into perennial organs whose part increases annually and those organs that live for just one growing season. The development of vegetative shoots and flowers at the periphery of the body of vegetation follows the same rules as that of annual organs. Buds have to be named instead of seeds since they, too, represent a resting state that has to be surmounted by an interaction of exogenous and endogenous factors.
The term homeosis was coined by BATESON in 1894. It describes the alternative developmental path of a certain organ. J. W. v. GOETHE discovered as soon as 1790 that plants consist of a number of homologous organs and that a progressive transformation that he called metamorphosis occurs in the course of their development beginning with their leaves and ending with parts of the flower. He assumed that the development was caused by a 'raw sap' that would increasingly be purified so that new organs could develop. He named the replacement of stamens by petals that can be observed in 'filled' flowers as an example. Gardeners have at all times invested a lot of work to select such varieties.
After the methods of molecular biology had been established arose again an interest in the development of Drosophila and a group of genes was discovered that decide whether and where the development of a certain compartment (wings, eyes, etc.) is initiated and whether an organ anlage can be transformed into that of another organ. These genes were called homeotic genes and today are they known to control the development of all pro- and eucaryotes (COEN, 1991; GASSER, 1991). In 1995 was C. NÜSSLEIN-VOLLHARD of the Max-Plank-Institut at Tübingen awarded the Nobel Prize for Medicine for her research on this topic. Homeotic genes act as switches at the level of transcription. They activate a set of genes required for the development of a certain organ en bloc.
It seemed to suggest itself to search for homeotic genes in plants,
too. The idea was fostered by the introduction of a new model
organism to molecular and developmental biology, the small and
inconspicuous crucifere Arabidopsis
thaliana (MEYEROWITZ and PRUITT, 1985). Under laboratory
conditions takes one generation a mere 4 - 5 weeks, it does not
need much space, and it has one of the smallest plant genomes
known. See the data base of Arabidopsis thaliana genes
for further information:
It is rather simple to yield a variety of mutants with an altered hormone metabolism or altered developmental steps. The genome's size is about 2 x 108 base pairs. At the time being work several research teams intensively at the complete elucidation of its nucleotide sequence and it can well be assumed that the work will be finished soon with the first complete genetic information of a plant genome.
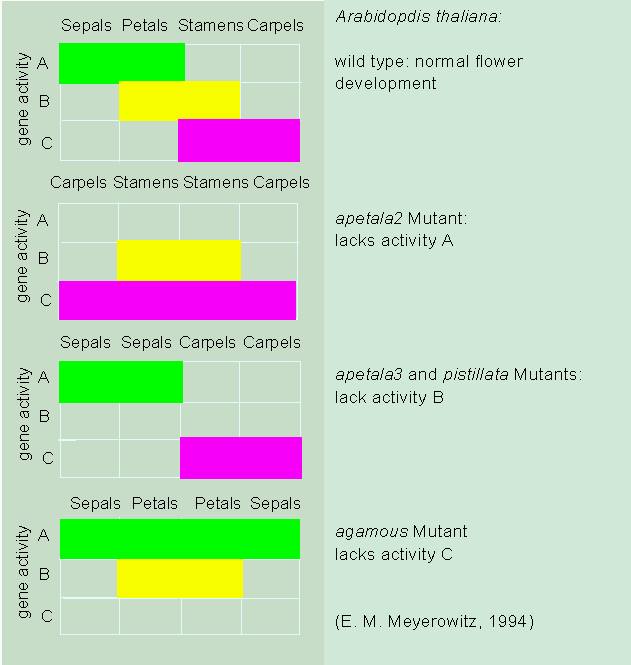
The work done in the lab of E. M. MEYEROWITZ (California Institute of Technology, Pasadena) concerning the determination of the single flower circles during anthesis (flower formation) is especially informative. The mutant apetala 3 and several others develop no petals and no stamens while the mutant agamous develops petals and sepals but neither stamens nor carpels. The genes of this group were termed MADS-box genes. The results of the mutant analysis can conclusively interpreted as follows: the formation of sepals and petals requires the activity of a gene called A, while that of petals and stamens requires a gene activity B and that of stamens and carpels one called C. That means that petals and stamens require two genes for their development and that the change from one flower circle to the next is based on the successive turning on of the respective switch. The team of H. SAEDLER at the Max Planck Institut für Züchtungsforschung at Cologne (SCHWARZ-SOMMER et al., 1990) yielded almost identical results with its research on Antirrhinum majus. The genetic results could be verified by the localization of the respective specific mRNA in the primordia of the single flower circles.
The MADS box is a highly conserved sequence motif found in a family of transcription factors. The conserved domain was recognized after the first four members of the family, which were MCM1, AGAMOUS, DEFICIENS and SRF (serum response factor). The name MADS was constructed form the "initials" of these four "founders"
MADS-box genes contribute significantly to the development of inflorescences and flowers, e.g. by acting as floral meristem or organ identity genes . Up to now, MADS-box genes have mainly been studied in the scientific context of developmental biology, and in eudicotyledonous model plants such as Antirrhinum and Arabidopsis. The focus of research tends to the application of current knowledge about flower development to breeding research and evolutionary biology. Therefore, currently the MADS-box gene family in other phylogenetic informative taxa is being characterized. Among these are monocots, such as the important crop plant maize (Zea mays ssp. mays), the ornamental plants lily (Lilium regale) and tulip (Tulipa gesneriana), and non-flowering plants, including the gymnosperm Gnetum gnemon, the ferns Ceratopteris and Ophioglossum, and the moss Physcomitrella patens.
For More Details see "The MADS-box Gene Home Page", developed and maintained by Günter Günter THEISSEN's Research Group at the Max-Planck-Institut für Züchtungsforschung in Cologne
Homeotic genes control, too, the normal development of a seedling (embryogenesis). The apical-basal pattern along the polarity axis or the radial pattern of primary tissue primordia as well as the changes in the primordias' shape are examples (work done in the lab of G. JÜRGENS, Lehrstuhl für Genetik, Universität München: MAYER, U. et al., 1991).
© Peter v. Sengbusch - b-online@botanik.uni-hamburg.de